Preparation method of salinomycin sodium granules
A technology of salinomycin and granules, which is applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, and pharmaceutical formulas, can solve problems such as increased production costs, waste of resources, etc., and achieves low cost and energy consumption. The effect of high production efficiency and low frequency of repeated granulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
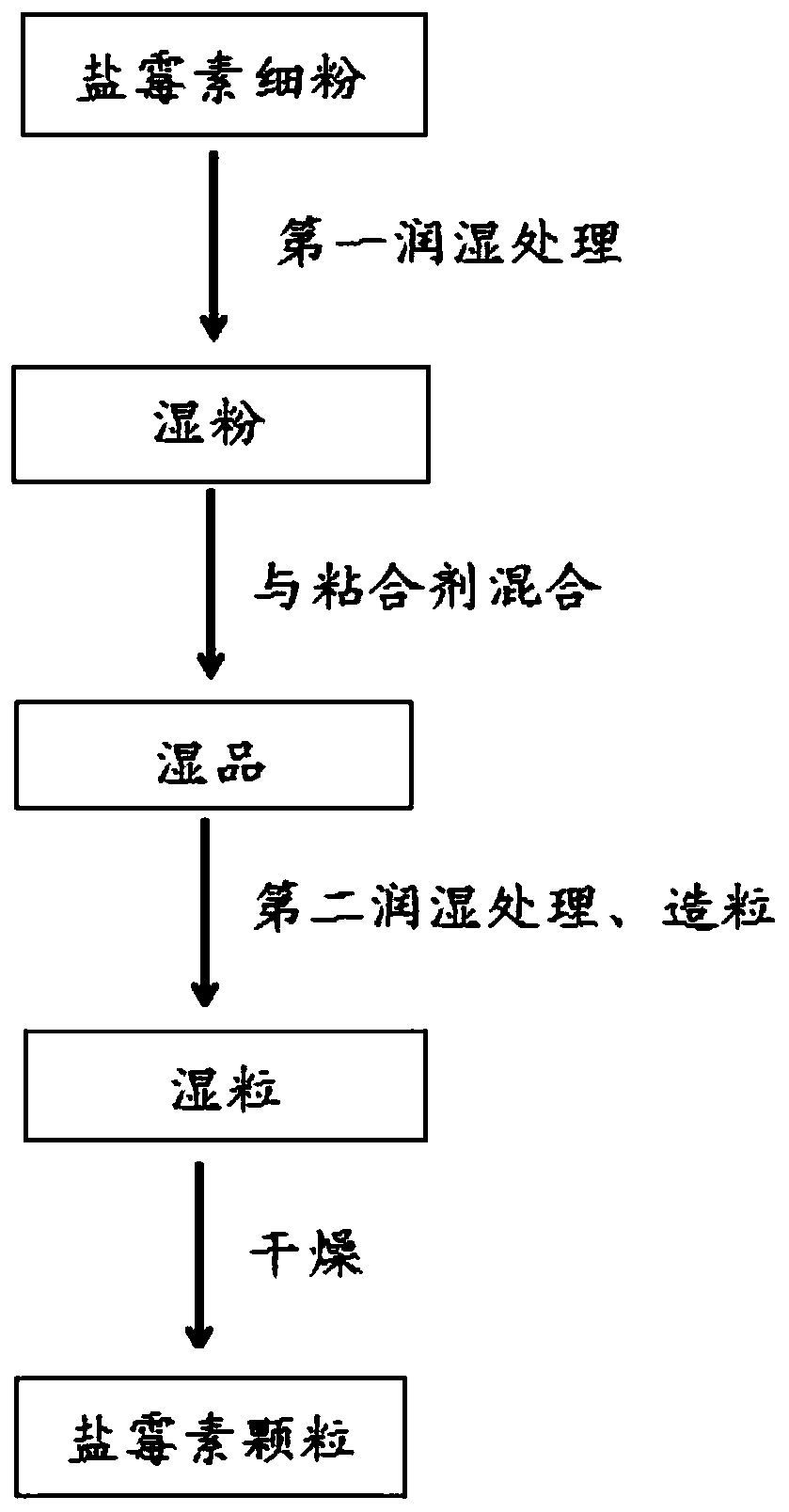
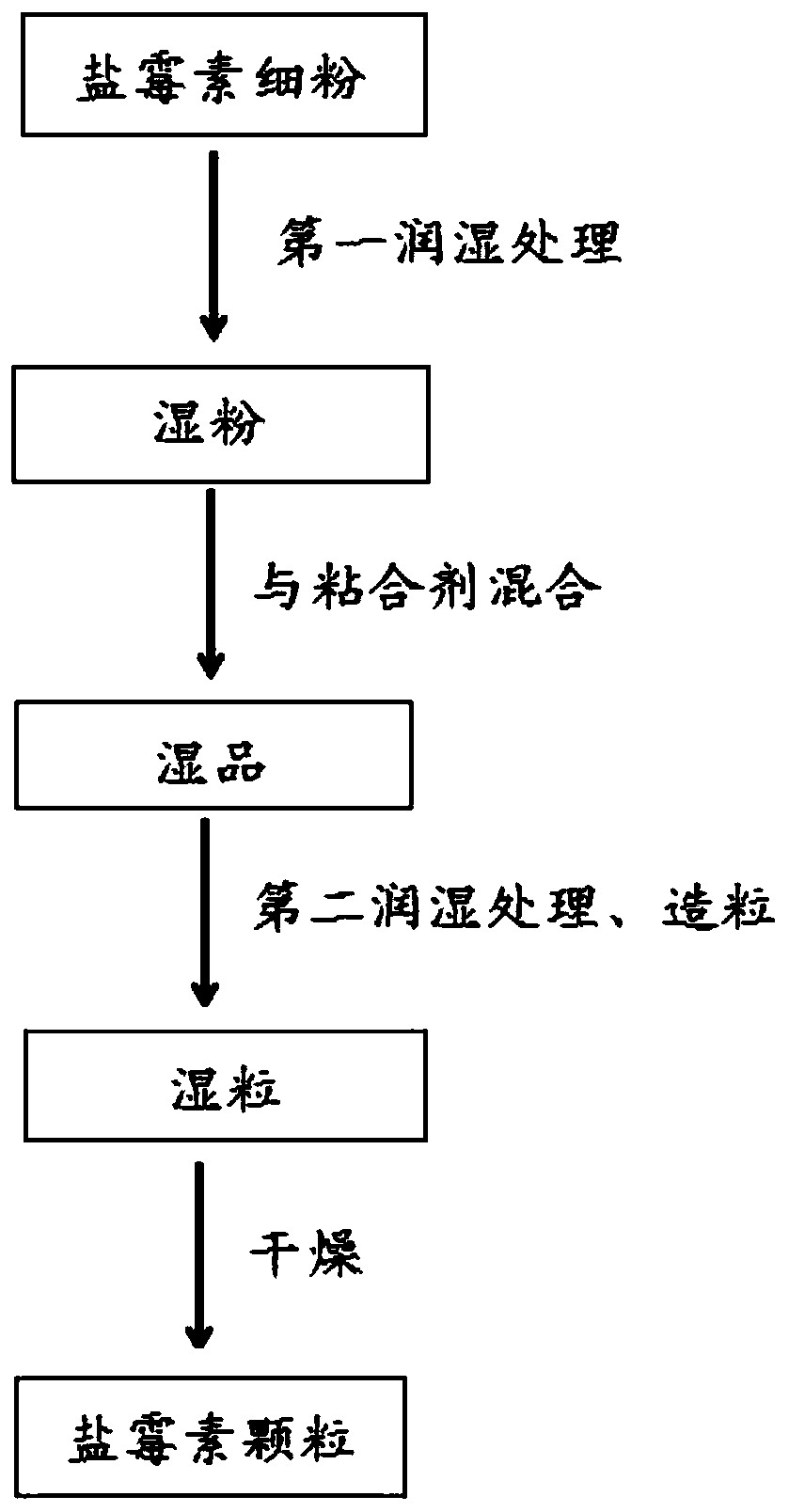
[0022] The invention provides a preparation method of salinomycin granules, comprising the following steps:
[0023] Mixing the fine powder of salinomycin with water and performing the first wetting treatment to obtain wet powder;
[0024] Mixing the wet powder and binder to obtain a wet product;
[0025] Mixing the wet product with water, performing the second wetting treatment, and then granulating to obtain wet granules;
[0026] The wet granules were dried to obtain salinomycin granules.
[0027] In the present invention, the salinomycin fine powder and water are mixed for the first wetting treatment to obtain the wet powder. In the present invention, the source of the salinomycin fine powder is preferably the fine powder sieved during the production process of salinomycin. In the present invention, the first wetting pretreatment is preferably carried out under stirring conditions, and the stirring speed is preferably 20-30 rpm, more preferably 23-28 rpm; the stirring t...
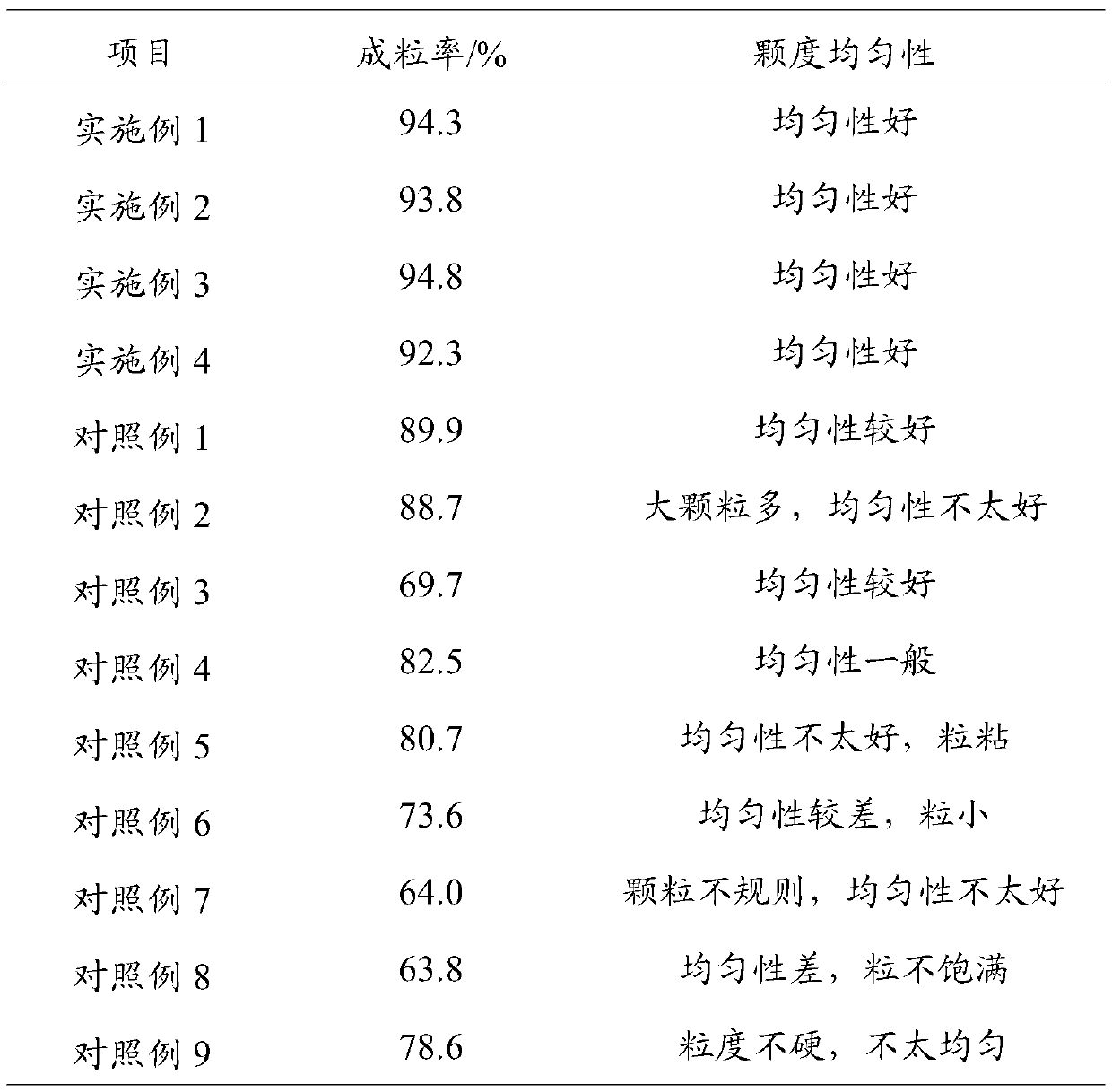
Embodiment 1
[0037] (1) 1000kg of salinomycin powder and water are placed in a coulter mixer, stirred for 15 minutes at 25 rpm, and the first wetting treatment is carried out to obtain a wet powder with a moisture content of 16.7%;
[0038] (2) Dissolve 10kg of dextrin in 100kg of water to obtain an aqueous solution of dextrin, mix the wet powder, 25kg of sodium lignosulfonate and 25kg of calcium lignosulfonate, add the aqueous solution of dextrin, and stir at 25rpm for 50min for mixing , get the wet product;
[0039] (3) The wet product and water are placed in a mixer and mixed with water for the second wet treatment to obtain a granulated material with a moisture content of 45%, and the obtained granulated material is added to a rotary granulator for granulation to obtain wet grain;
[0040] (4) Send the wet granules into a fluidized dryer via a conveyor belt for drying for 45 minutes, wherein the temperature parameters of the fluidized dryer are as follows: the inlet air temperature is...
Embodiment 2
[0042] (1) 1000kg of salinomycin powder and water are placed in a coulter mixer, stirred at 25rpm for 10min, and the first wetting treatment is carried out to obtain a wet powder with a moisture content of 20%;
[0043] (2) Dissolve 10kg of dextrin in 100kg of water to obtain an aqueous solution of dextrin, mix the wet powder, 30kg of sodium lignosulfonate and 25kg of calcium lignosulfonate, add the aqueous solution of dextrin, and stir at 25rpm for 50min for mixing , get the wet product;
[0044] (3) The wet product and water are placed in a mixer and mixed with water for the second wet treatment to obtain a granulated material with a moisture content of 45%, and the obtained granulated material is added to a rotary granulator for granulation to obtain wet grain;
[0045] (4) Send the wet granules into a fluidized dryer via a conveyor belt for drying for 45 minutes, wherein the temperature parameters of the fluidized dryer are as follows: the inlet air temperature is 110-120°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com