Method for preparing sulindac
A technology of sulindac and indene acetic acid is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., and can solve problems such as cumbersome operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
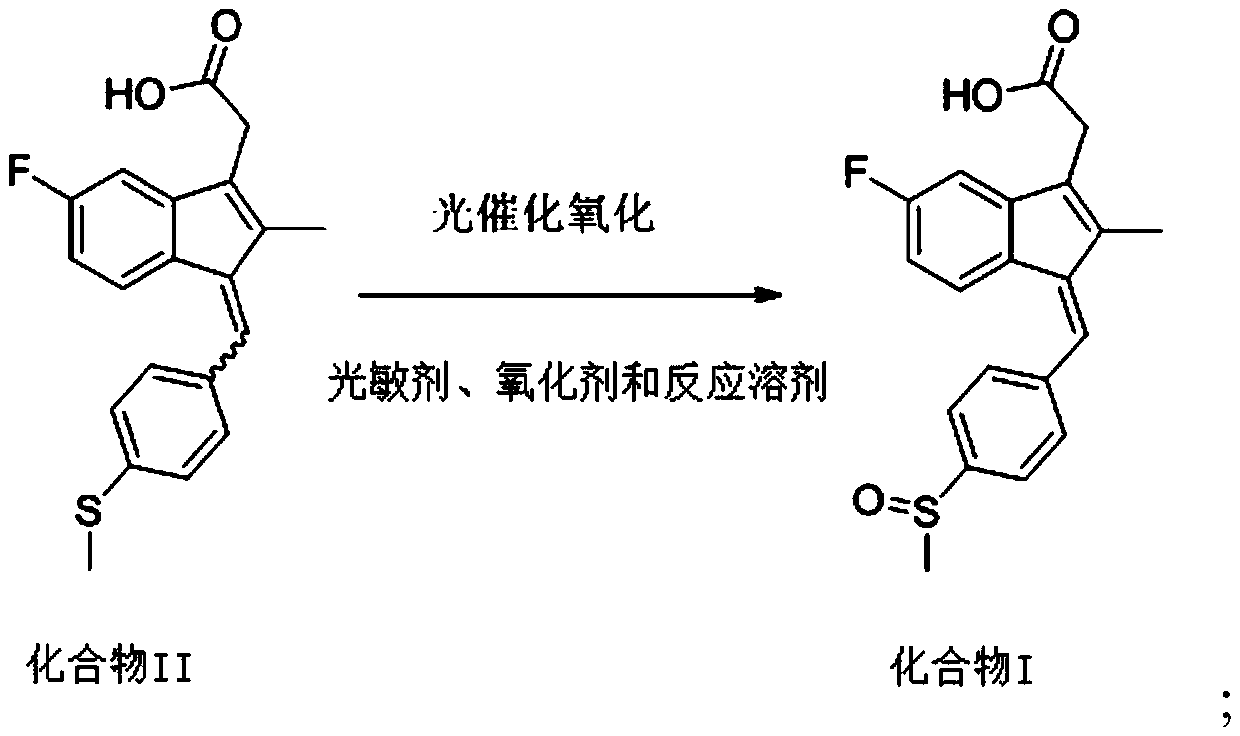
[0022] The invention provides a kind of preparation method of sulindac, comprising the following steps:
[0023] Mix 5-fluoro-2-methyl-1-(4-methylthiobenzylidene)-3-indene acetic acid, photosensitizer, oxidizing agent and reaction solvent, and carry out oxidation reaction under ultraviolet light irradiation conditions to obtain sulindac acid;
[0024] Wherein, the 5-fluoro-2-methyl-1-(4-methylthiobenzylidene)-3-indene acetic acid includes E-type isomer and Z-type isomer.
[0025] The preparation method of sulindac provided by the invention directly uses 5-fluoro-2-methyl-1-(4-methylthiobenzylidene)-3- Indene acetic acid is obtained as a raw material through an oxidation reaction to obtain sulindac without the need for repeated recrystallization and The reaction is transformed and the operation is simple. In the present invention, the proportion of the Z-type isomer in the 5-fluoro-2-methyl-1-(4-methylthiobenzylidene)-3-indeneacetic acid is preferably 50-92%. The present in...
Embodiment 1
[0041] Add 25g of 5-fluoro-2-methyl-1-(4-methylthiobenzylidene)-3-indene to a 1L reaction vessel equipped with a thermometer, a stirrer and a UV light source (wavelength range 315-400nm) Acetic acid (Z:E=66:33), 5.6g peroxyacetic acid, 0.5g anthracene and 300g ethanol were reacted at 25°C and 300r / min for 5h under the condition of ultraviolet light irradiation; after the reaction was completed, evaporated from the obtained system 250g of ethanol was cooled to 10°C for recrystallization, and suction filtered to obtain 25.5g of sulindac with a yield of 97.4% and a purity greater than 99.5%.
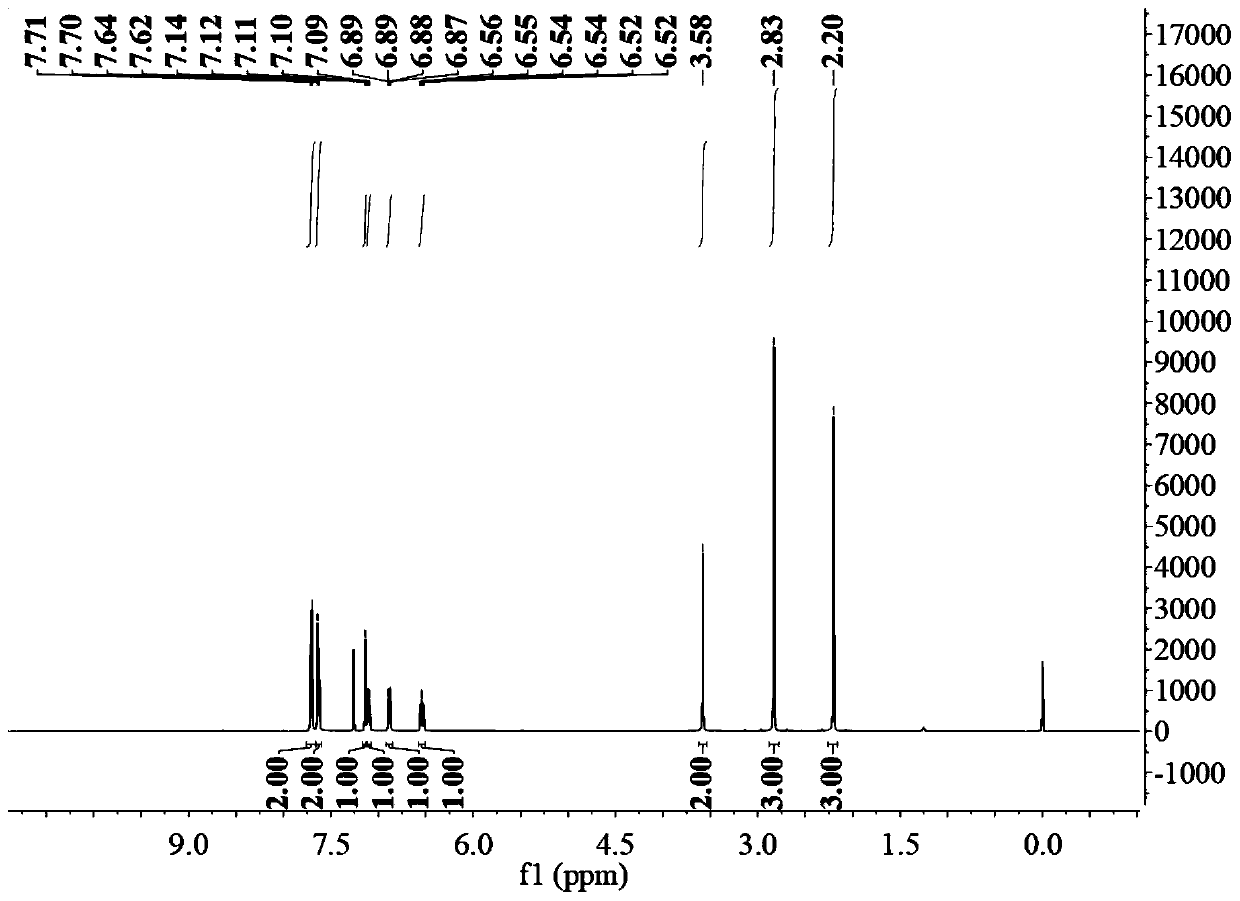
[0042] figure 1 For the proton nuclear magnetic spectrum of the product prepared in this embodiment, the data are as follows:
[0043] 1 HNMR (500MHz, CDCl 3 )δ7.71(d, J=8.2Hz, 2H), 7.63(d, J=8.2Hz, 2H), 7.14(s, 1H), 7.11(dd, J=8.4, 5.1Hz, 1H), 6.88( dd,J=8.8,2.3Hz,1H),6.54(td,J=8.9,2.3Hz,1H),3.58(s,2H),2.83(s,3H),2.20(s,3H).
[0044] Depend on figure 1 It can be seen from the above d...
Embodiment 2
[0046] Add 25g of 5-fluoro-2-methyl-1-(4-methylthiobenzylidene)-3-indene to a 1L reaction vessel equipped with a thermometer, a stirrer and a UV light source (wavelength range 315-400nm) Acetic acid (Z:E=66:33), 6.15g peroxyacetic acid, 0.5g anthracene and 375g toluene were reacted at 10°C and 300r / min for 12h under the condition of ultraviolet light irradiation; after the reaction was completed, the obtained system was evaporated to dryness, The residue was dissolved in 50 g of ethanol, cooled to 10°C for recrystallization, and suction filtered to obtain 25.9 g of sulindac with a yield of 98.9% and a purity greater than 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com