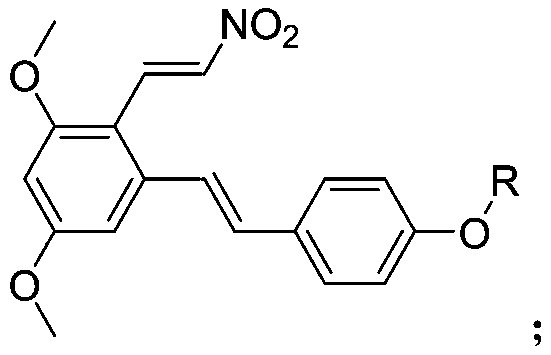
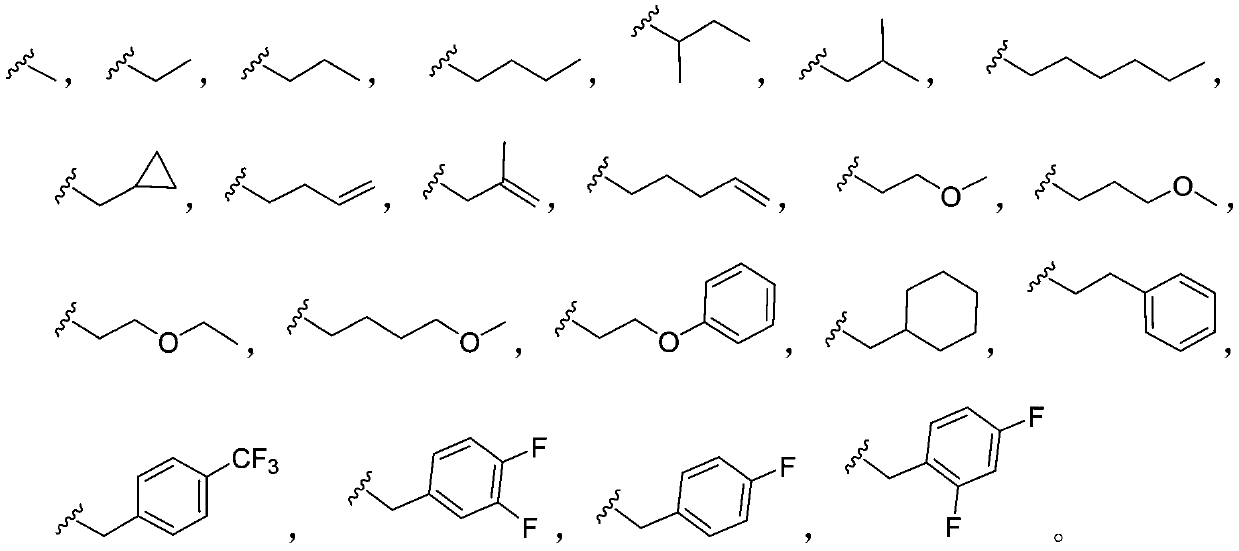
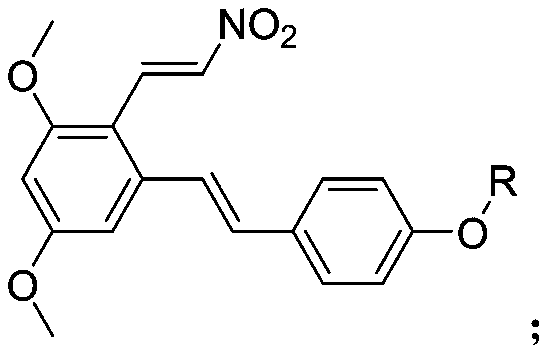
3-4'-5-trihydroxystilbene nitroethylene compound as well as preparation method and application thereof
A technology of resveratrol and nitroethylene, applied in the field of resveratrol nitroethylene compounds and preparation thereof, can solve the problems of poor selectivity, low bioavailability, poor water solubility of resveratrol, etc. Inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of 1,5-dimethoxy-3-((E)-4-(methoxy)styryl)-2-(E)-nitrovinyl)benzene (compound 1)
[0030]
[0031] Weigh pterostilbene A (1.28g, 5mmol) and dissolve it in a 100mL round bottom flask with acetone, add anhydrous potassium carbonate (1.38g, 6mmol), tetrabutylammonium bromide (1.94g, 6mmol), methyl bromide (380uL , 6mmol), reflux reaction, TLC plate to monitor the reaction process, after the completion of the reaction, remove potassium carbonate by suction filtration, vacuum concentration, column chromatography separation (ethyl acetate:petroleum ether=1:5), to obtain white solid B.
[0032] Weigh B (811mg, 3mmol) and dissolve it in a 100mL round bottom flask with acetonitrile, add N,N-dimethylformamide (DMF, 458μL), then slowly add phosphorus oxychloride (280μL, 3mmol); dropwise After completion, return to room temperature for reaction, stir for 1 hour, and stop immediately after the reaction of the system is complete. Take a 1000mL beaker, add 500mL ice wa...
Embodiment 2
[0035] Preparation of 1,5-dimethoxy-3-((E)-4-(ethoxy)styryl)-2-(E)-nitrovinyl)benzene (compound 2)
[0036]
[0037] The preparation method is the same as in Example 1. Substituting ethyl bromide for methyl bromide, the title compound was obtained as a yellow solid powder with a yield of 91% and a melting point of 149-153°C. 1 H NMR (600MHz, DMSO-d 6)δ8.31(d, J=13.3Hz, 1H), 7.89(d, J=13.2Hz, 1H), 7.58(d, J=8.6Hz, 2H), 7.41(dd, J=16.1, 7.9Hz, 1H), 7.05(d, J=16.0Hz, 1H), 6.95(d, J=8.6Hz, 2H), 6.85(d, J=1.9Hz, 1H), 6.63(s, 1H), 4.04(q, J=6.9Hz, 2H), 3.94(d, J=5.3Hz, 3H), 3.89(s, 3H), 1.32(t, J=7.0Hz, 3H). 13 C NMR (151MHz, CDCl 3 (C 20 h 22 NO 5 ,[M+H] + ).
Embodiment 3
[0039] Preparation of 1,5-dimethoxy-2-((E)-2-nitrovinyl)-3-((E)-4-(propoxy)styryl)benzene (compound 3)
[0040]
[0041] The preparation method is the same as in Example 1. Propane bromide was used instead of methyl bromide to obtain the target compound as a yellow solid powder with a yield of 91% and a melting point of 100-102°C. 1 H NMR (600MHz, CDCl 3 )δ8.40(d, J=13.3Hz, 1H), 7.91(d, J=13.3Hz, 1H), 7.46(d, J=8.1Hz, 2H), 7.25(d, J=16.0Hz, 1H) ,6.94-6.88(m,3H),6.72(s,1H),6.42(s,1H),3.96(t,J=6.5Hz,2H),3.93(s,3H),3.90(s,3H), 1.86-1.79(m,2H),1.06(t,J=7.4Hz,3H). 13 C NMR (151MHz, CDCl 3 )δ165.89,136.95,135.15,130.90,125.89,117.55,107.18,100.22,72.30,58.42,58.21,32.32,25.20,13.12.MS(ESI):392.1648.(C 21 h 23 NO 5 ,[M+Na] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com