Monooxygenase mutant and application thereof
A technology of monooxygenase and mutants, applied in the direction of application, oxidoreductase, and introduction of foreign genetic material using vectors, etc., can solve the problems of low enzyme activity and poor selectivity of monooxygenase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
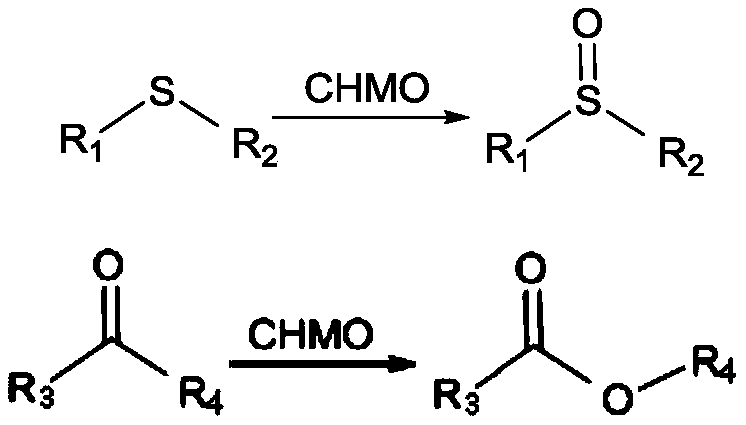
Method used
Image
Examples
Embodiment approach
[0050] According to a typical embodiment of the present invention, a recombinant plasmid is provided. The recombinant plasmid contains any one of the above DNA molecules. The DNA molecules in the above recombinant plasmids are placed in appropriate positions of the recombinant plasmids, so that the above DNA molecules can be replicated, transcribed or expressed correctly and smoothly.
[0051] Although the qualifier used in the present invention is "contains" when limiting the above-mentioned DNA molecule, it does not mean that other sequences irrelevant to its function can be arbitrarily added to both ends of the DNA sequence. Those skilled in the art know that in order to meet the requirements of recombination operations, it is necessary to add suitable restriction endonuclease cutting sites at both ends of the DNA sequence, or additionally add start codons, stop codons, etc., therefore, if using A closed-ended statement will not truly cover these situations.
[0052] The ...
Embodiment 1
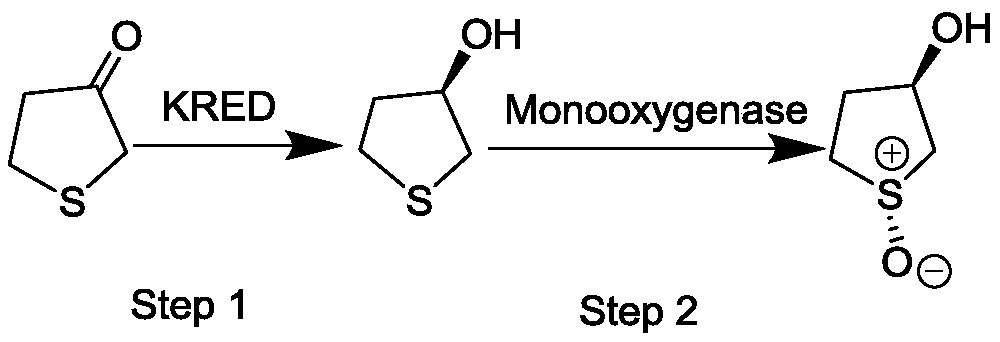
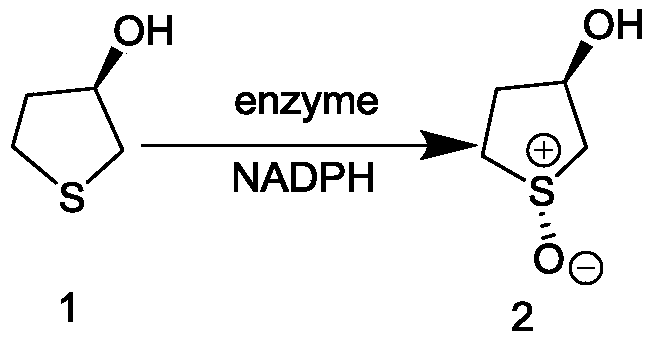
[0066] Comparison of Reaction Properties of (R,R)-1-Oxo-3-Hydroxytetrahydrothiophene Prepared by Site-directed Mutagenesis of Monooxygenase
[0067] In a 50mL glass conical flask, add 100mg (R)-3-hydroxytetrahydrothiophene to 850μL isopropanol, mix well, adjust the pH to 6.0-9.0, then add 800mg of monooxygenase, isopropanol Dehydrogenase 0.12g, 50μL (20mg / mL) NADP + , in the crude enzyme solution of 0.1M Tris-HCl buffer, the total reaction volume is 10mL, the system pH is 6.0-9.0, and the reaction is carried out in a constant temperature shaker flask at 15°C-30°C. After 16 hours, take 700 μL of the reaction system, add 1.4 mL of absolute ethanol, centrifuge at 12,000 rpm for 5 min, add 0.5 g of anhydrous MgSO to the supernatant 4 Remove water, centrifuge at 12000rpm for 5min, take supernatant and use N 2 After drying, re-dissolve with 700 μL absolute ethanol and send to GC for analysis. The response characteristics of some mutants are shown in Table 1:
[0068] Table 1
...
Embodiment 2
[0073] Comparison of Reaction Properties of (R,R)-1-Oxo-3-Hydroxytetrahydrothiophene Prepared by Combined Mutation of Monooxygenase
[0074] In a 50mL glass conical flask, add 100mg (R)-3-hydroxytetrahydrothiophene to 850μL isopropanol, mix well, adjust the pH to 6.0-9.0, then add 800mg of monooxygenase, isopropanol Dehydrogenase 0.12g, 50μL (20mg / mL) NADP + , in the crude enzyme solution of 0.1M Tris-HCl buffer, the total reaction volume is 10mL, the system pH is 6.0-9.0, and the reaction is carried out in a constant temperature shaker flask at 15°C-30°C. After 16 hours, take 700 μL of the reaction system, add 1.4 mL of absolute ethanol, centrifuge at 12,000 rpm for 5 min, add 0.5 g of anhydrous MgSO to the supernatant 4 Remove water, centrifuge at 12000rpm for 5min, take supernatant and use N 2 After drying, re-dissolve with 700 μL absolute ethanol and send to GC for analysis. The response characteristics of some mutants are shown in Table 2:
[0075] Table 2
[0076] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com