Recombinant escherichia coli expressing alpha-L-rhamnosidase and application of recombinant escherichia coli
A technology for recombining Escherichia coli and rhamnosidase is applied in the fields of genetic engineering and biomedicine, and can solve the problems of restricting industrial production and utilization, long fermentation time, difficulty in separation and purification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 AnRhaE gene synthesis and recombinant expression of α-L rhamnosidase Escherichia coli construction
[0036] According to the codon optimization of the rhamnoside hydrolase AnRhaE gene of Aspergillus nidulans in the NCBI database, the gene synthesis was performed by Jinweizhi Company, and the final gene sequence is shown in SEQ ID NO.2.
[0037]The primer pair designed to amplify the AnRhaE sequence was F: TTA AGA AGG AGA TAT ACC ATG GCA ATGTCT TTG TCT ATA TCT GGT GT, R: GTG CGG CCG CAA GCT TGT CGACAC CTAAAG TTG ATTCGA ATC TAT. The synthetic sequence was used as a template, and the primer pair was used for PCR amplification, and the Primer Star MasterMix (Takara company) high-fidelity pfu enzyme was selected for pre-denaturation at 95°C for 3 minutes; the amplification stage was 30 cycles, followed by 95°C for 10s , 53°C, 5s, 72°C, 2min; extension 72°C, 5min. The PCR product was purified, and the vector pET28a was subjected to Nco I and Sal I double enzyme dig...
Embodiment 2
[0038] Example 2 Inducing recombinant Escherichia coli to ferment and produce α-L rhamnosidase
[0039] Streak the constructed recombinant Escherichia coli BL21(DE3) / pET28a-AnRhaE (using the strain BL21(DE3) / pET28a transformed with an empty vector as a control) on an LB plate containing a kanamycin concentration of 50 μg / mL, 37 Cultivate at ℃ for 12h. Pick a single colony and transfer it into 5 mL of LB liquid medium containing kanamycin at a concentration of 50 μg / mL, and shake at 220 rpm for 12 hours at 37°C. Transfer to 100 mL of TB liquid medium containing 50 μg / mL of kanamycin according to the inoculum size of 1%, and shake at 220 rpm at 37°C until OD 600 The value is 0.8. Isopropylthiogalactopyranoside (IPTG) was added to the culture to a final concentration of 0.5 μmol / L, and the shaking culture was continued at 25° C. and 220 rpm for 16 h.
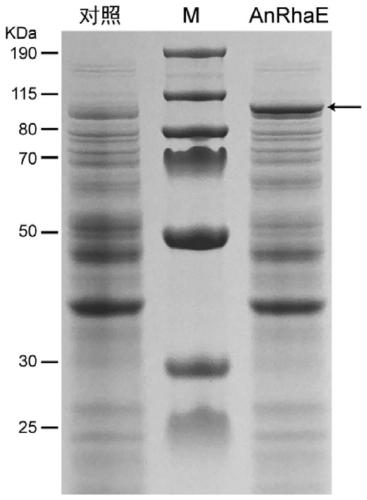
[0040] At the end of the culture, draw 1 mL of the culture to measure the final OD 600 The cells were collected by centrifuga...
Embodiment 3
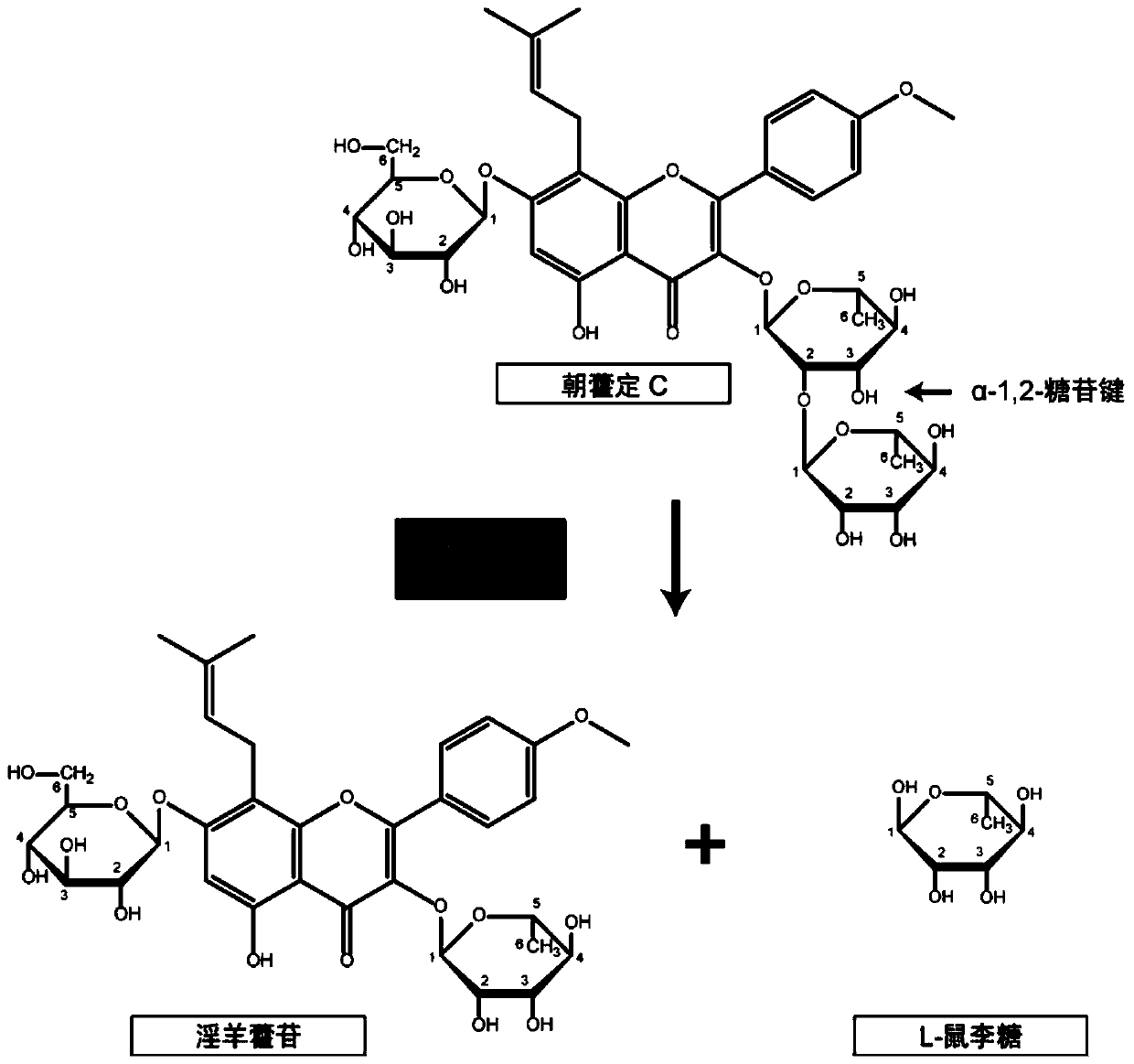
[0041] Example 3 Recombinant AnRhaE Hydrolyzes Epimedin C Rhamnoside-α-1,2-Rhamnoside Bond
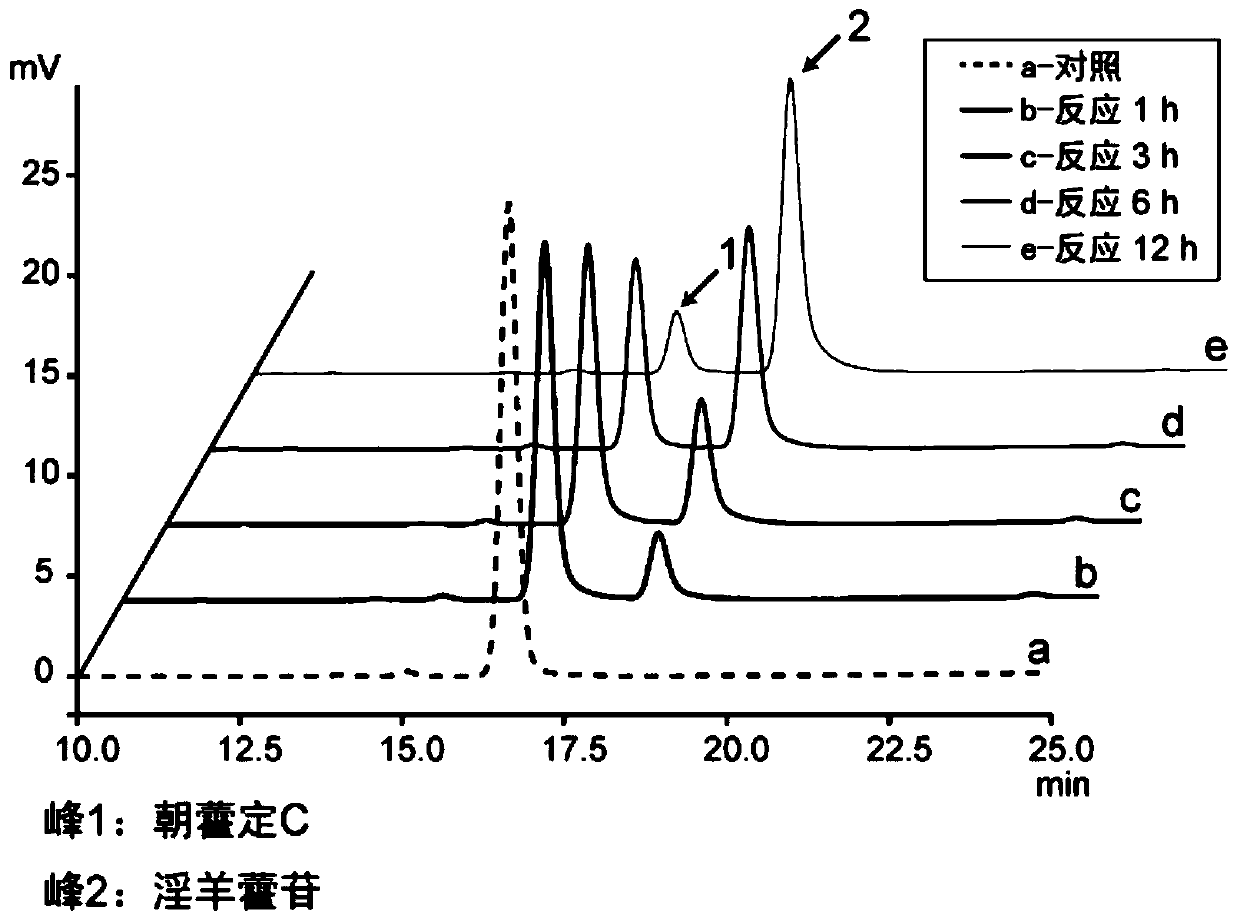
[0042] The recombinant α-L-rhamnosidase enzyme liquid was prepared, and the enzyme activity was measured by pNPR. Pipette 40 μL of recombinant α-L-rhamnosidase enzyme solution into a 1.5 mL centrifuge tube, add 10 μL of Epimedin C solution (concentration 1 g / L, dissolved in absolute ethanol), and add 150 μL of deionized water. The catalytic reaction was carried out at 37°C, and 200 μL of absolute ethanol was added to the centrifuge tube every 60 minutes to terminate the reaction. The reaction solution was centrifuged at 12000 × g for 10 min, filtered through a 0.22 μm organic filter membrane, and analyzed by HPLC (see figure 2 ). Recombinant α-L-rhamnosidase has the activity of hydrolyzing the 3-position rhamnoside-α-1,2-rhamnoside bond of Epimedin C. When the added activity is 460U / L, it can completely catalyze the hydrolysis of 1g / L in 90min Epimedin C produces icariin (see ima...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com