Method for detecting related substances in imidafenacin
A detection method and related substance technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of certain risks in drug quality assessment, degradation products that have not been analyzed and detected, and impurities that are easily interfered by solvent peaks. Achieve the effect of good separation, more types and numbers of impurities, and avoid missed detection and less detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
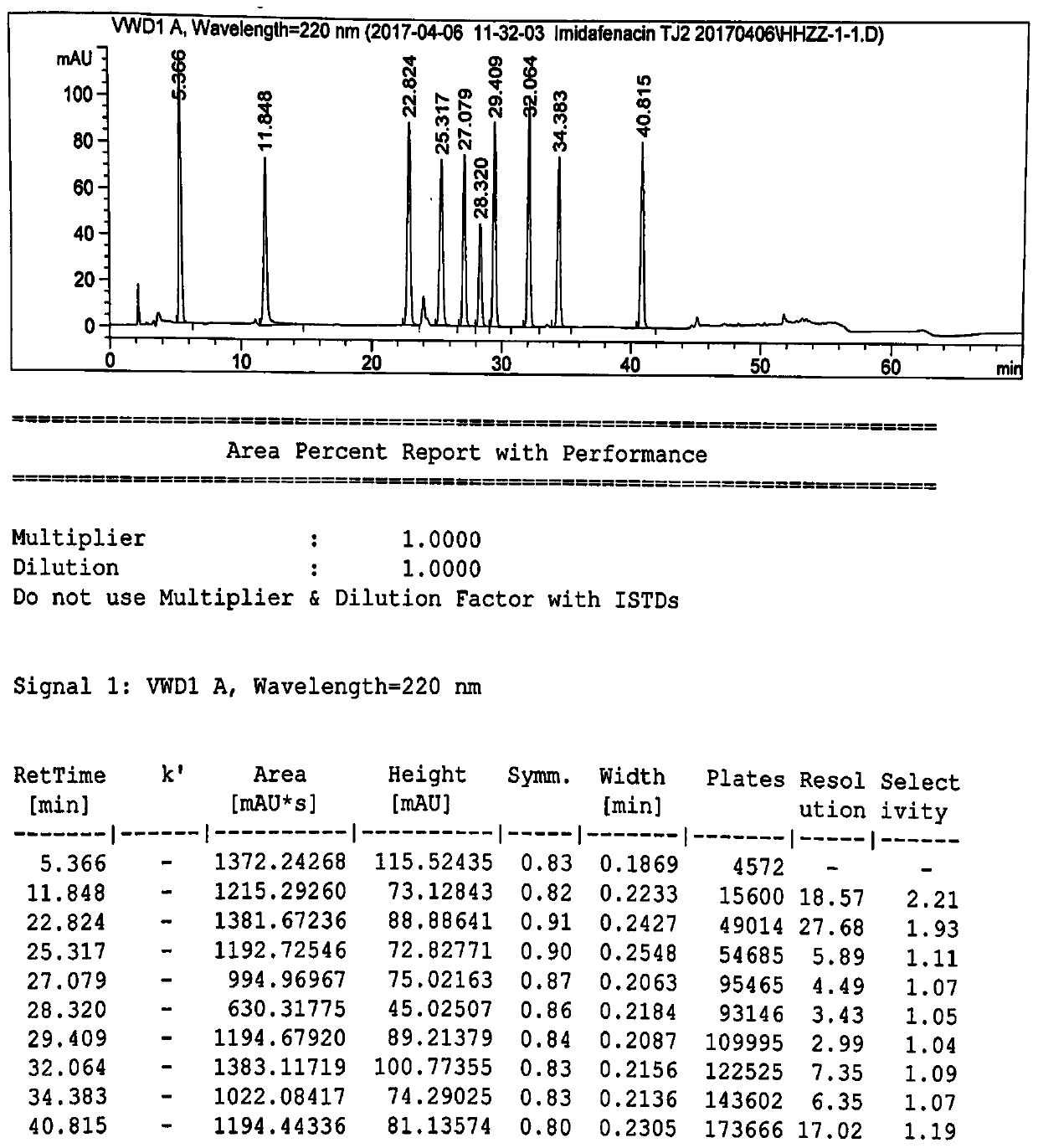
[0044] High performance liquid chromatography conditions:
[0045] The chromatographic column is an octadecylsilane bonded silica gel column, the model is Inertsil ODS-3C18 (250×4.6mm, 5μm), and the phosphoric acid solution of sodium octane sulfonate (take 1.08g sodium octane sulfonate, add mass concentration Dissolve and dilute to 1000ml for 0.1% phosphoric acid solution, adjust the pH value to 2.8 with triethylamine) as mobile phase A, with acetonitrile as mobile phase B, gradient elution, flow velocity is 1.0ml / min, detection wavelength is 220nm, column The temperature was 25°C, and the injection volume was 100 μl. Solvent 1 is 70% acetonitrile water, and solvent 2 is a mixed solution of mobile phase A and mobile phase B with a volume ratio of 67:33.
[0046] The gradient elution program is: (1) in 0-40 minutes, the volume ratio of mobile phase A and mobile phase B changes gradually from 80:20 to 60:40 at a uniform speed; (2) in 40-50 minutes, mobile phase A and mobile pha...
Embodiment 2
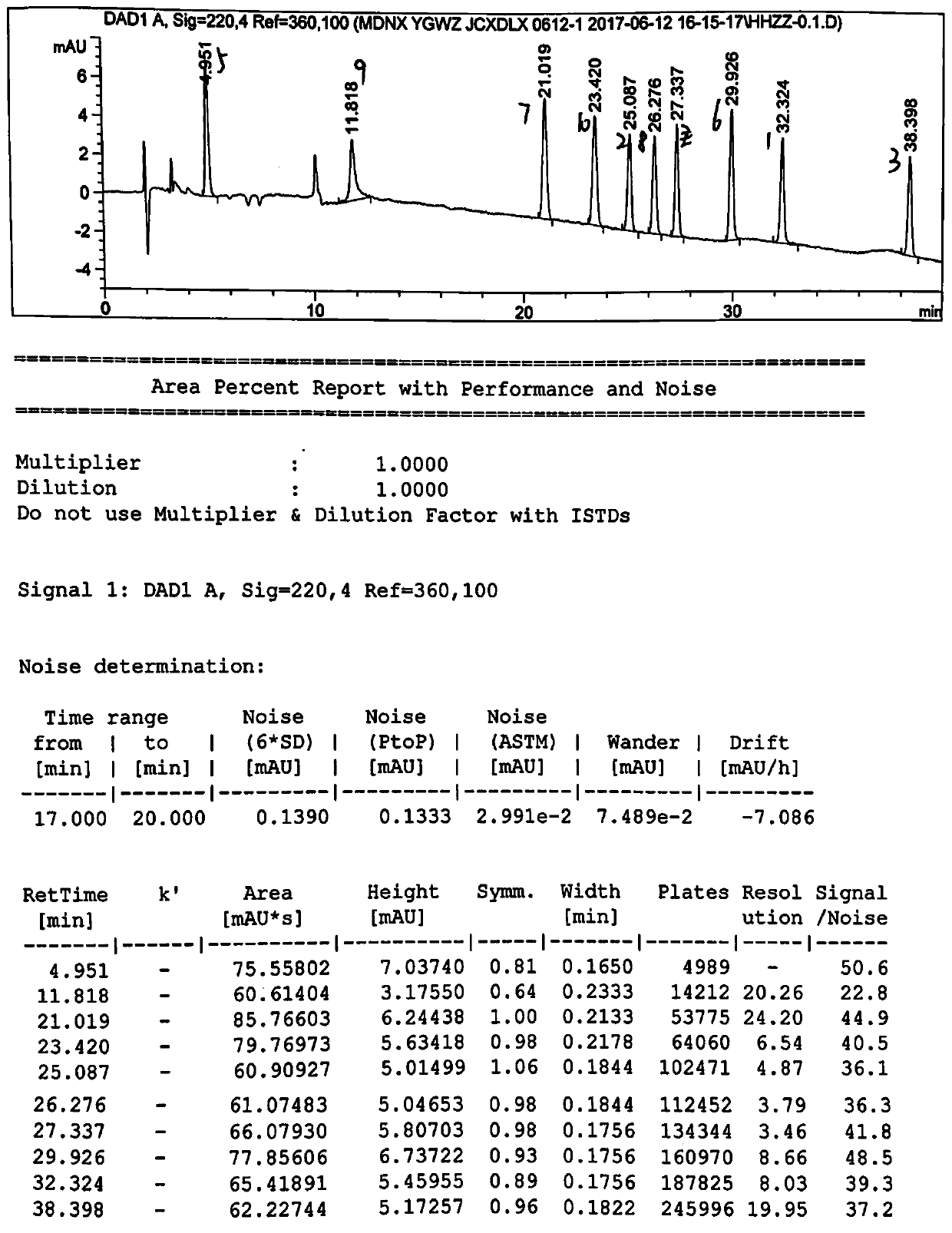
[0051] High performance liquid chromatography conditions:
[0052] The chromatographic column is an octadecylsilane bonded silica gel column, the model is Inertsil ODS-3C18 (250×4.6mm, 5μm), and the phosphoric acid solution of sodium octane sulfonate (take 1.08g sodium octane sulfonate, add mass concentration Dissolve and dilute to 1000ml for 0.1% phosphoric acid solution, adjust the pH value to 2.8 with triethylamine) as mobile phase A, with acetonitrile as mobile phase B, gradient elution, flow velocity is 1.0ml / min, detection wavelength is 220nm, column The temperature was 25°C, and the injection volume was 50 μl. Solvent 1 is 70% acetonitrile water, and solvent 2 is a mixed solution of mobile phase A and mobile phase B with a volume ratio of 67:33.
[0053] The gradient elution program is: (1) in 0-40 minutes, the volume ratio of mobile phase A and mobile phase B changes gradually from 80:20 to 60:40 at a uniform speed; (2) in 40-50 minutes, mobile phase A and mobile phas...
Embodiment 3
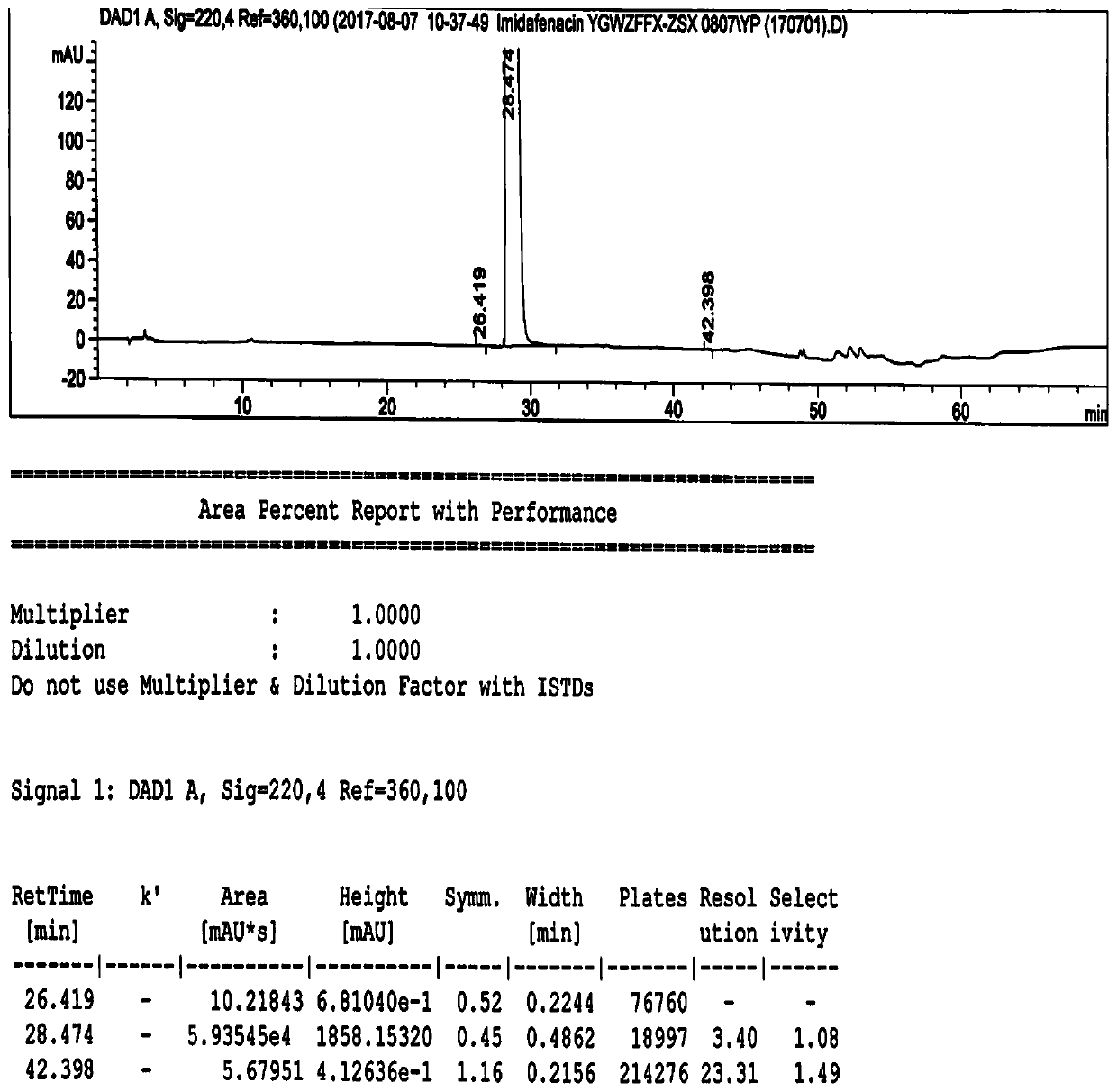
[0118] High performance liquid chromatography conditions:
[0119] The chromatographic column is an octadecylsilane bonded silica gel column, the model is Inertsil ODS-3C18 (250×4.6mm, 5μm), and the phosphoric acid solution of sodium octane sulfonate (take 1.08g sodium octane sulfonate, add mass concentration Dissolve and dilute to 1000ml for 0.1% phosphoric acid solution, adjust the pH value to 2.8 with triethylamine) as mobile phase A, with acetonitrile as mobile phase B, gradient elution, flow velocity is 1.0ml / min, detection wavelength is 218nm and 222nm, column temperature 25°C, injection volume 50μl. Solvent 1 is 70% acetonitrile water, and solvent 2 is a mixed solution of mobile phase A and mobile phase B with a volume ratio of 67:33.
[0120] The gradient elution program is: (1) in 0-40 minutes, the volume ratio of mobile phase A and mobile phase B changes gradually from 80:20 to 60:40 at a uniform speed; (2) in 40-50 minutes, mobile phase A and mobile phase B The vo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com