Amantadine hapten and preparing method and application thereof
A technology of amantadine and hapten, which is applied in the preparation of cyanide reaction, chemical instruments and methods, and the preparation of organic compounds, to achieve the effects of optimizing reaction time, optimizing reaction temperature, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
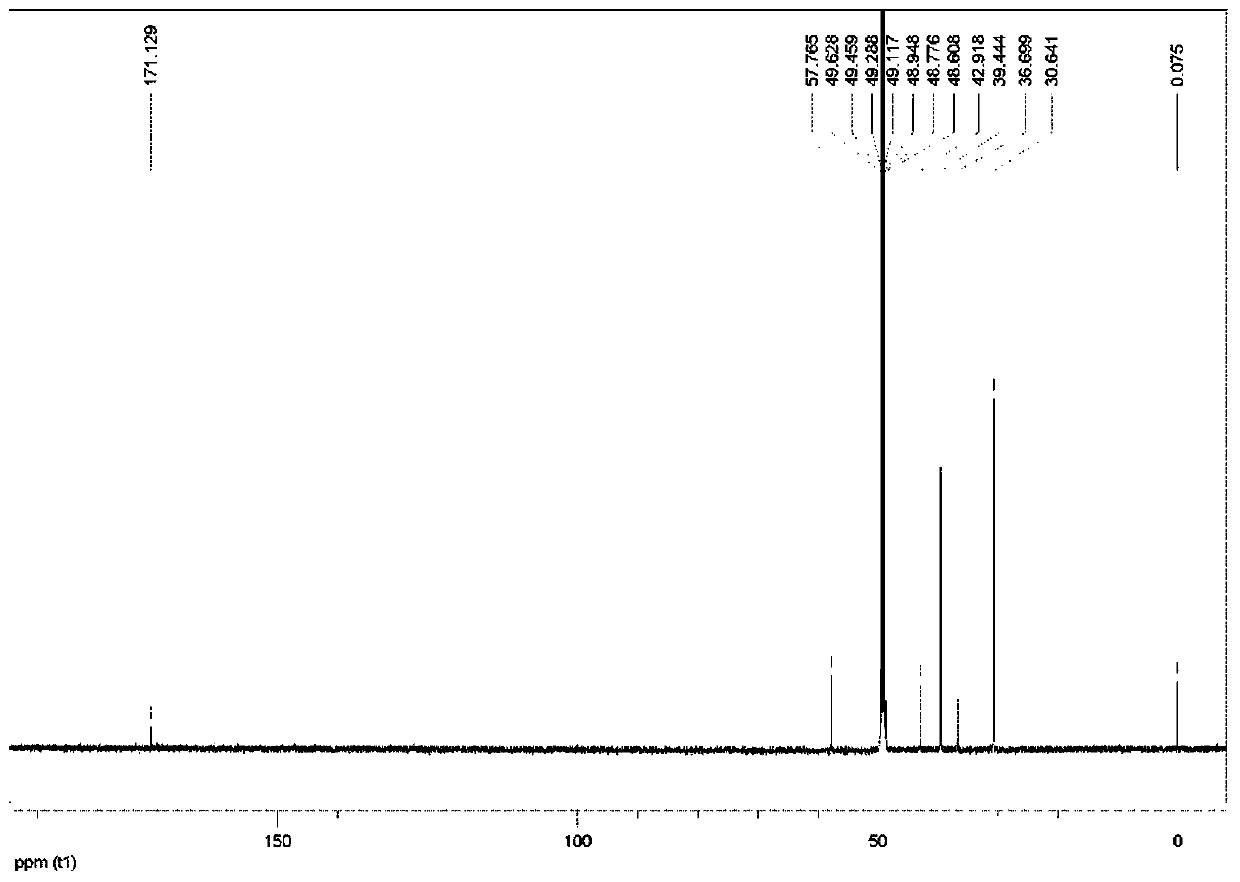
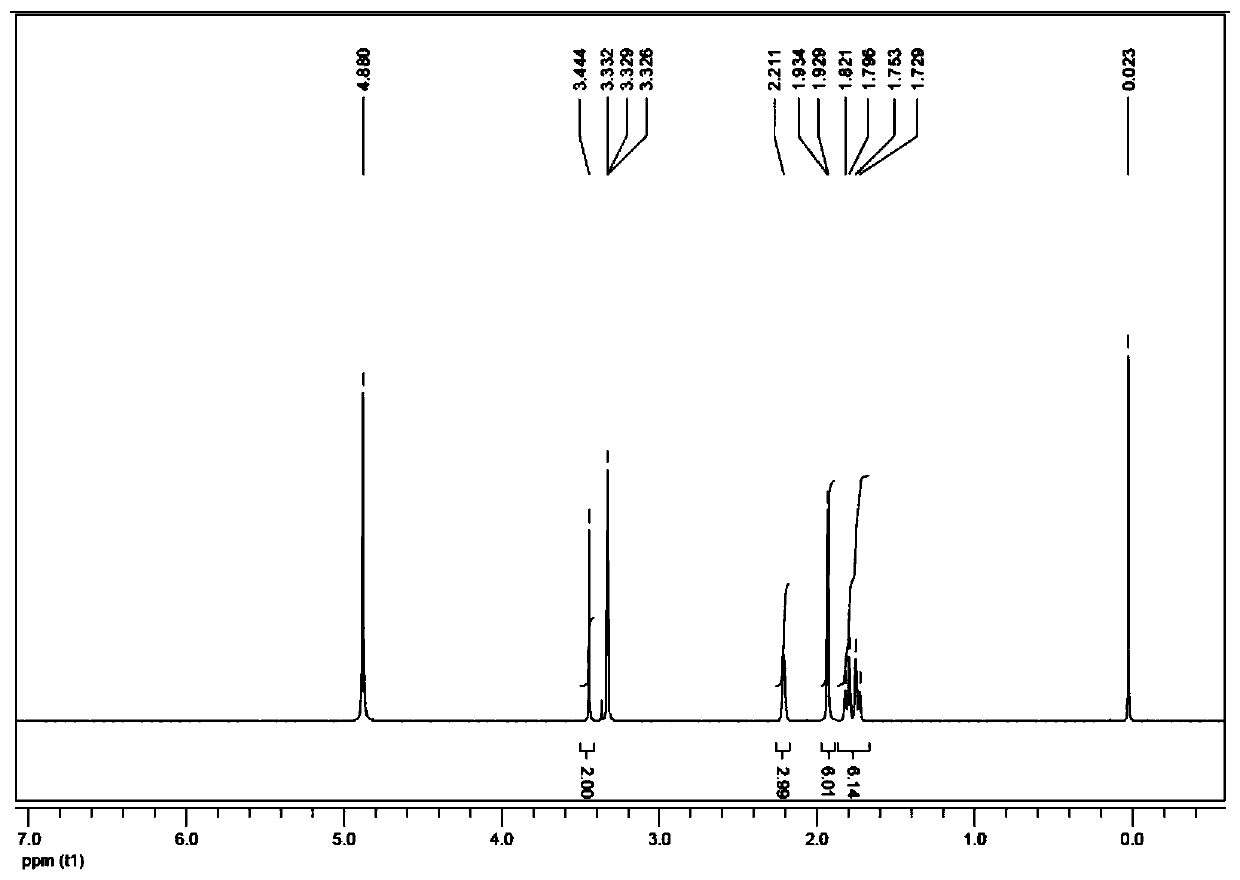
[0045] Preparation of 2-(adamantyl)aminoacetic acid:
[0046] a) Preparation of ethyl 2-(adamantyl)aminoacetate: Dissolve 3.04 g of amantadine in 20 mL of DMF, add 3.9 g of ethyl bromoacetate and 5.5 g of K 2 CO 3 , stirred and reacted at 60°C for 16 hours; then added 50mL of water to dilute, and then extracted with ethyl acetate (3×50mL); after the organic phase was combined, it was dried with sodium, and then the solid was filtered out, and the filtrate was concentrated in vacuo and purified by a silica gel column Can obtain ethyl 2-(adamantyl) aminoacetate;
[0047]b) Preparation of 2-(adamantyl)aminoacetic acid: Dissolve 2g of ethyl 2-(adamantyl)aminoacetate in aqueous ethanol (ethanol:water=20:15), add 0.7g NaOH, and stir for 1 hour ; After ethanol was distilled off under reduced pressure, the pH of the solution was adjusted to 5 with 3N HCl; the mixture was concentrated and purified in vacuo to finally obtain 2-(adamantyl)aminoacetic acid.
[0048] c) The synthesis pr...
Embodiment 2
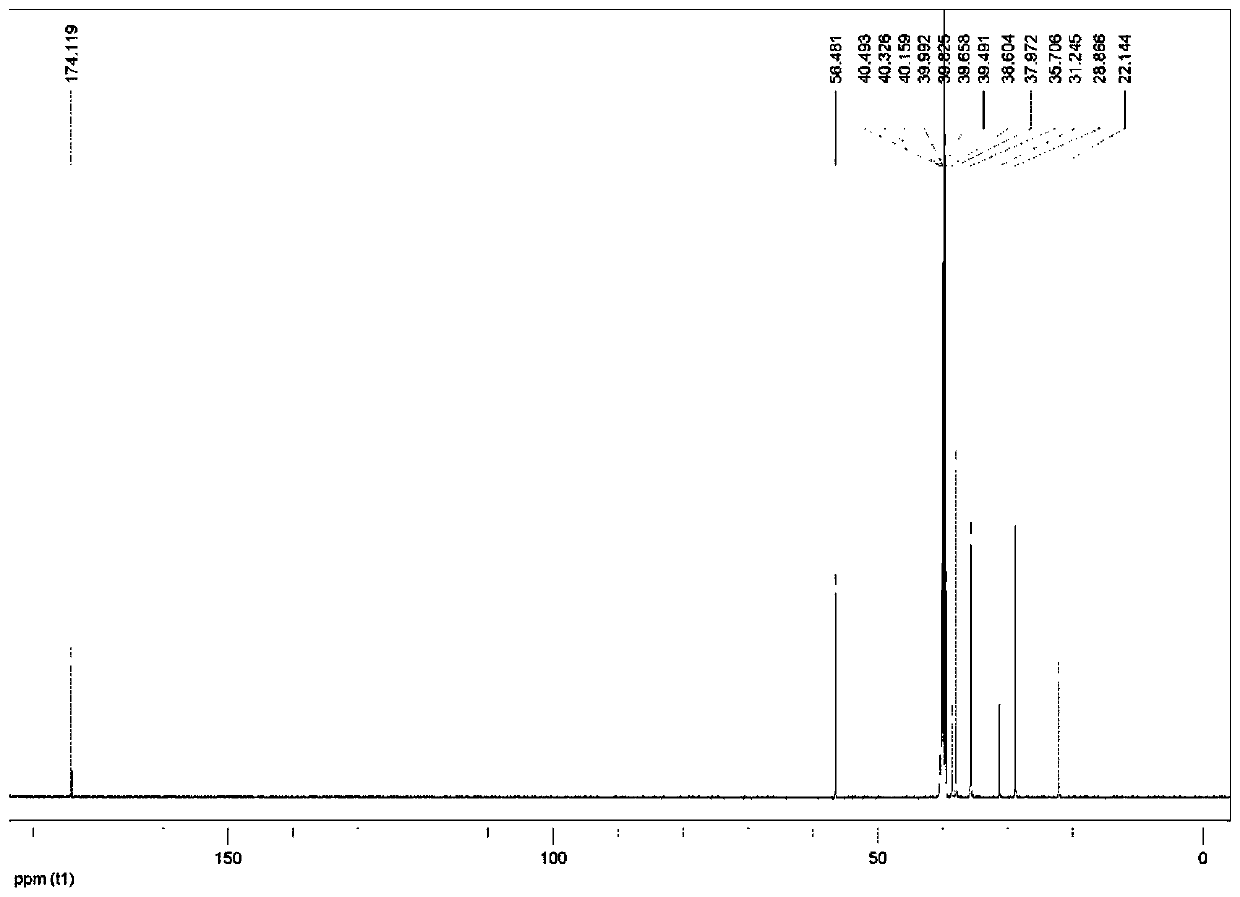
[0052] Preparation of 4-(adamantyl)aminobutyric acid:
[0053] a) Preparation of ethyl 4-(adamantyl)aminobutyrate: Dissolve 5.6g ethyl 4-bromobutyrate in 15mL DMF, add 4.4g amantadine and 8.1g K 2 CO 3 , stirred and reacted at 60°C for 16 hours; then added 100mL of water to dilute, and then extracted with ethyl acetate (3×50mL); after the organic phase was combined, it was dried with sodium, and then the solid was filtered out, and the filtrate was concentrated and purified in vacuo. Obtain ethyl 4-(adamantyl) aminobutyrate;
[0054] b) Preparation of 4-(adamantyl)aminobutyric acid: Dissolve 3g ethyl 4-(adamantyl)aminobutyrate in aqueous ethanol (ethanol:water=20:15), add 0.9g NaOH, and stir for reaction 1 hour; after the ethanol was distilled off under reduced pressure, the pH of the solution was adjusted to 5 with 3N HCl; the mixture was concentrated and purified in vacuo to finally obtain 4-adamantanemethylaminobutyric acid.
[0055] c) The synthesis process steps are as...
Embodiment 3
[0059] Preparation of 8-(adamantyl)aminocaprylic acid:
[0060] a) Preparation of ethyl 8-(adamantyl)aminooctanoate: Dissolve 5.04g amantadine in 15mL DMF, add 9.1g ethyl bromooctanoate and 9.1g K 2 CO 3 , stirred and reacted at 60°C for 16 hours; then added 100mL of water to dilute, and then extracted with ethyl acetate (3×50mL); after the organic phase was combined, it was dried with sodium, and then the solid was filtered out, and the filtrate was concentrated and purified in vacuo. Obtain ethyl 8-(adamantyl)aminooctanoate;
[0061] b) Preparation of 8-(adamantyl)aminocaprylic acid: Dissolve 4g of ethyl 8-(adamantyl)aminocaprylic acid in aqueous ethanol (ethanol:water=1:1), add 0.9g NaOH, and stir for 1 hour ; After removing ethanol, the pH of the solution was adjusted to 5 with 3N HCl; the mixture was concentrated and purified in vacuo to finally obtain 8-(adamantyl)aminocaprylic acid.
[0062] c) The synthesis process steps are as follows:
[0063]
[0064] d) Resu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com