Synthesis method of On-DNA aryl azide compound in construction of DNA coding compound library
A technology of compound library and aryl azide, which is applied in the field of DNA-encoded compound library, can solve the problems such as the scarcity and limitation of binary reagents, and achieve the effects of convenient operation, mild reaction conditions, and cheap and easy-to-obtain catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
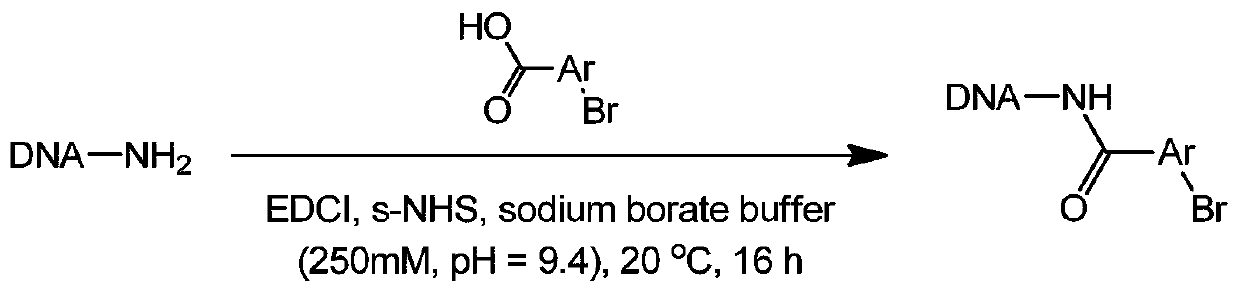
[0047] Embodiment 1, the synthesis of On-DNA aryl halogenated compound
[0048] DNA-NH 2 (For example, the starting head fragment mentioned in the patent CN108070009A) was dissolved in 250mM, pH=9.5 boric acid buffer solution, prepared into a 1mM concentration solution, distributed into a 96-well plate, and used EDCI with the halogenated aryl carboxyl binary compound As a condensing agent and s-NHS condensation activator, the corresponding On-DNA aryl halogenated compound (references: Nat.Chem., 2015, 7, 3, 241, see figure 1 ), after the reaction is completed, only ethanol precipitation treatment is performed, concentrated and dried, and directly used for the next reduction reaction.
[0049]
Embodiment 2
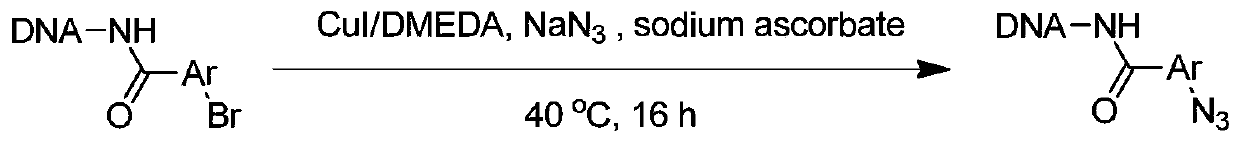
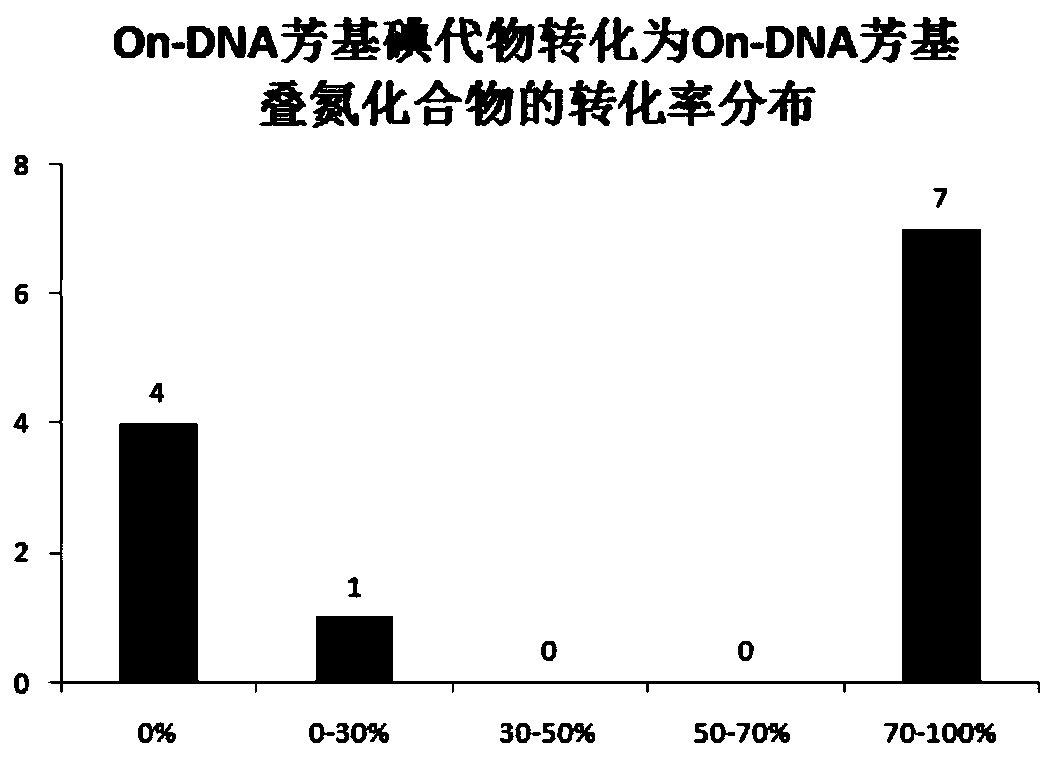
[0050] Embodiment 2, the synthesis of On-DNA aryl azides
[0051] The DNA-Ar-X generated in situ was redissolved in ultrapure water, prepared into a 1mM concentration solution, dispensed into a new 96-well plate (10μL, 10nmol, 1mM aqueous solution), and sequentially added sodium azide solution (25μL , 5000nmol, 200mM aqueous solution, 500eq.), CuI solution (10μL, 300nmol, 30mM dimethyl sulfoxide solution, 300eq.), N,N'-dimethylethylenediamine solution (10μL, 300nmol, 30mM di Methyl sulfoxide solution, 600eq.), sodium ascorbate solution (5μL, 1000nmol, 200mM aqueous solution, 300eq.), centrifuged, let the solution sink to the bottom, vortex mixed, centrifuged again, replaced nitrogen 3 times, sealed Membranes and 96-well plates were reacted in a PCR instrument at 40°C for 16 hours (cover temperature: 40°C).
[0052]
[0053] Copper removal:
[0054] After the reaction is complete, add sodium diethyldithiocarbamate solution (6.0μL, 600nmol, 100mM aqueous solution, 60eq.) to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com