MSC (mesenchymal stromal cells) precision medicine marker combination for screening and target treatment of patients with ARDS (acute respiratory distress syndrome)
A technology for treating drugs and patients, applied in the field of MSC precision medicine marker combination, can solve problems such as adverse effects, achieve the effect of preventing harmful effects and enhancing clinical treatment effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
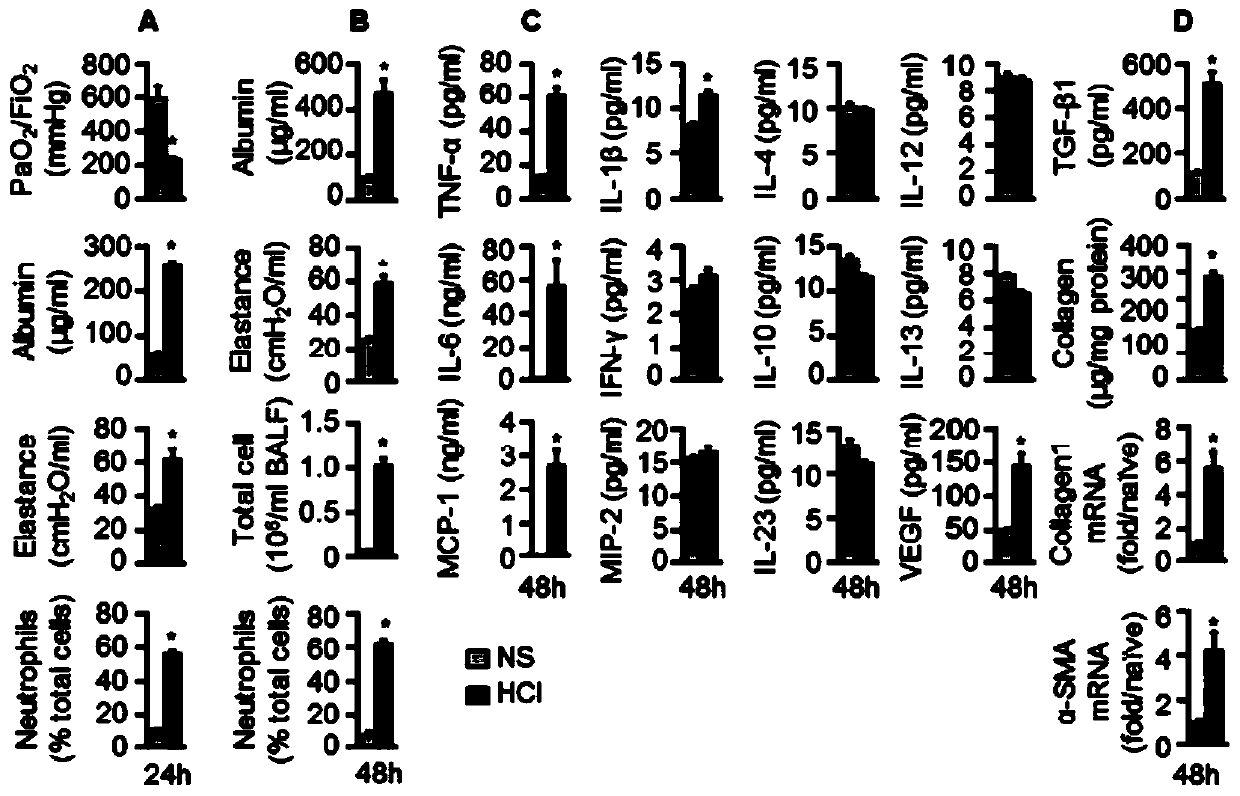
[0134] Example 1 Construction of mouse lung injury model and its response to MSC administration
[0135] 1. Construction of mouse lung injury model
[0136] 1. Experimental method
[0137] Male C57BL / 6 mice of 14-16 weeks were anesthetized and intubated, then infused with HCl (1N, pH 1) or normal saline (NS) at 3ml / kg, and mechanically ventilated for 10 minutes to make the instilled liquid in the lungs evenly distributed within.
[0138] Mice were anesthetized 48 hours after instillation and reintubated for low-pressure (LP) or high-pressure (HP) mechanical ventilation, and mice that retained spontaneous breathing (SB) were used as controls.
[0139] A total of 6 groups of mouse models were obtained:
[0140] (1) HCl+SB;
[0141] (2) NS+SB;
[0142] (3) HCl+LP;
[0143] (4) NS+LP;
[0144] (5) HCl+HP;
[0145] (6) NS+HP.
[0146] The physiological and biochemical indicators and gene protein expression of the mouse model were detected.
[0147] 2. Experimental results...
Embodiment 2
[0169] The pulmonary microenvironment characterization factor of embodiment 2 human ARDS patient
[0170] 1. Experimental method
[0171] To further investigate the relevance of the findings observed in mice, key proteins were examined in the plasma of ARDS and cardiac patients within 48 hours of admission in the ICU or CCU.
[0172] 2. Experimental results
[0173] A proteomic pattern similar to that present in mouse models was observed in ARDS patients ( Figure 12 A), with very good consistency.
[0174] The total antioxidant capacity, IL-6 and fibronectin concentrations in the lung microenvironment of CCU and ARDS patients were used to make 3D scatter plots. The microenvironment in the lungs of CCU and ARDS patients has different characteristics, and the average area (yellow box) of the three factors in patients with mild ARDS is used as a reference range to screen out ARDS patients who are suitable for MSC treatment ( Figure 12 B). Therefore, understanding the lung ...
Embodiment 3
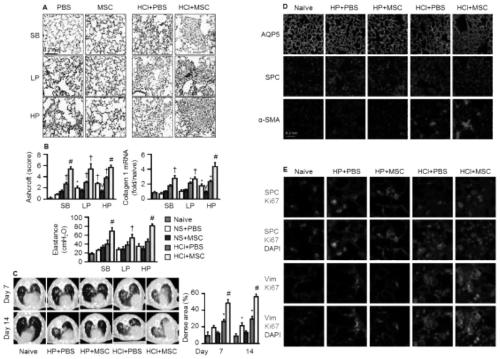
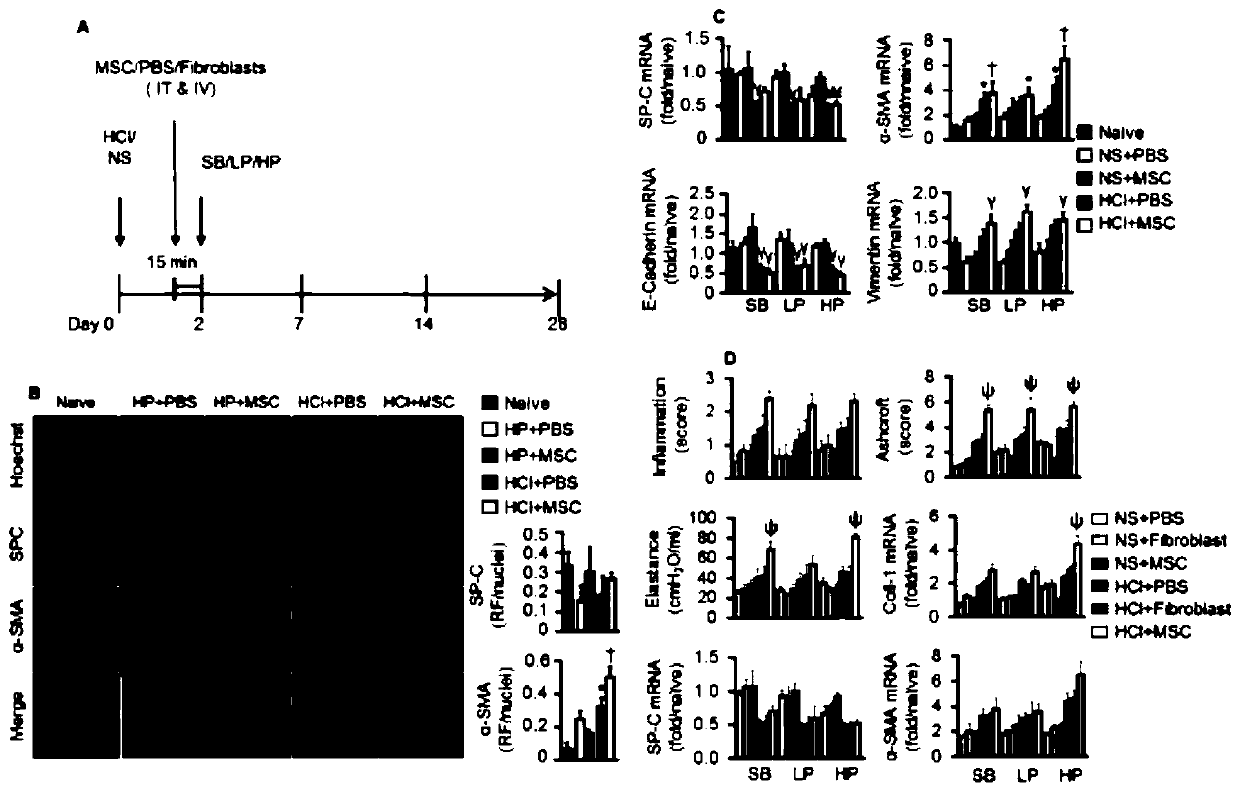
[0179] Administration of embodiment 3 genetically modified MSCs
[0180] 1. Experimental method
[0181] In order to overcome the adverse effects produced by MSCs and avoid harmful lung microenvironment (i.e. pro-fibrotic, procoagulant and pro-inflammatory), we will carry human hepatocyte growth factor (LV HGF ) or human interleukin-10 (LV IL-10 ) lentivirus transiently transformed MSC to obtain human hepatocyte growth factor (MSC HGF ) or human interleukin-10 (MSC IL-10 ) MSC.
[0182] carrying hIL-10 (MSC IL-10 ) or hHGF (MSC HGF ) MSCs were administered after HCl treatment for 48 hours, carrying a luciferase reporter gene (MSC Luc ) MSCs and PBS as controls.
[0183] Specific administration method: intratracheal cell administration (IT) is carried out first, followed by intravenous cell administration (IV) 15 minutes later.
[0184] The physiological and biochemical indicators and gene protein expression of BALF and lung tissue homogenate were detected.
[0185] 2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com