Recombinant spider silk protein and application thereof
A technology of spidroin and protease, which is applied in the protein field, can solve the problems of ignoring the degradability of spidroin protein, achieve the effect of ensuring mechanical properties or biological quality, wide application, and improving application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
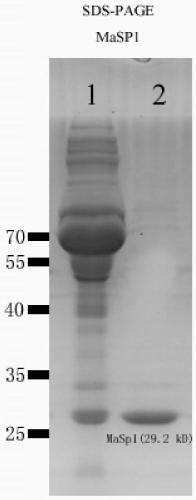
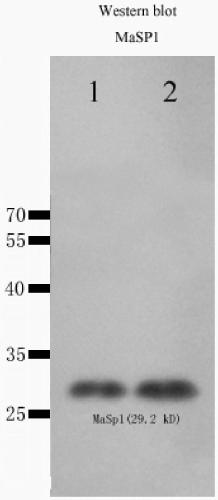
[0049] Example 1 Artificially synthesized recombinant spidroin gene MaSp1
[0050] The sequence structure of the recombinant spidroin protein selected in this embodiment is [T 1 -W n -T 2 -W n ] m , where n=1, m=4, T 1 The amino acid sequence of the spidroin domain monomer is shown in SEQ ID NO.1, T 2 The amino acid sequence of the spidroin protein domain monomer is shown in SEQ ID NO.2, the amino acid sequence of the protease cleavage site of W is KKKKSSSSSESSRSSSSSSS (the letters above are common amino acid abbreviations), and the recombinant spidroin protein The amino acid sequence is shown in SEQ ID NO.13. According to the recombinant spidroin protein sequence selected above and the principle of Escherichia coli preferred codon usage, a nucleotide sequence encoding a recombinant spidroin protein consistent with the amino acid sequence of the recombinant spidroin protein was designed , see the nucleotide sequence of SEQ ID NO.14 in the sequence listing.
Embodiment 2
[0051] Example 2. High expression of the gene MaSp1 of artificially synthesized recombinant spidroin in Escherichia coli
[0052] (1) The MaSp1 gene was synthesized by a gene synthesis company, cloned into the prokaryotic expression vector pET-28a+ containing a strong T7 promoter, and constructed into a recombinant plasmid pET-28a+-MaSp1 containing the artificially synthesized MaSp1 gene;
[0053] (2) Prepare Escherichia coli BL21(DE3) competent cells, and transform the recombinant plasmid pET-28a+-MaSp1 into the host cell BL21(DE3) by heat shock method (heat shock at 42°C for 45 seconds) to obtain the engineered plasmid containing the recombinant plasmid strains;
[0054] (3) Inoculate the engineered strain into the LB culture medium solution, culture it on a shaker at 37°C at 220rpm, when the concentration OD600 of the engineered strain reaches 0.6-0.8, add 0.5mmol / L IPTG, induce expression at 37°C for 6 hours, and then Lysis was carried out, and the resulting cell lysate w...
Embodiment 3
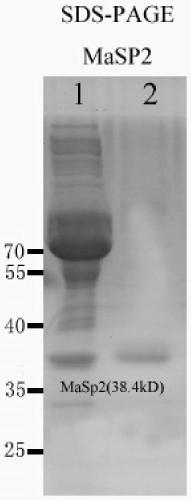
[0055] Example 3 Artificially synthesized recombinant spidroin gene MaSp2
[0056] The sequence structure of the recombinant spidroin protein selected in this embodiment is [T 1 -W n -T 2 -W n ] m , where n=1, m=4, T 1 The amino acid sequence of the spidroin domain monomer is shown in SEQ ID NO.3, T 2 The amino acid sequence of the spidroin protein domain monomer is shown in SEQ ID NO.4, the amino acid sequence of the protease cleavage site of W is ENLYFQS (the letters above are common amino acid abbreviations), and the recombinant spidroin protein The amino acid sequence is shown in SEQ ID NO.15. According to the recombinant spidroin protein sequence selected above and the principle of using preferred codons in Escherichia coli, the nucleotide encoding the recombinant spidroin protein consistent with the amino acid sequence of the recombinant spidroin protein was designed For the sequence, see the nucleotide sequence of SEQ ID NO.16 in the sequence listing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com