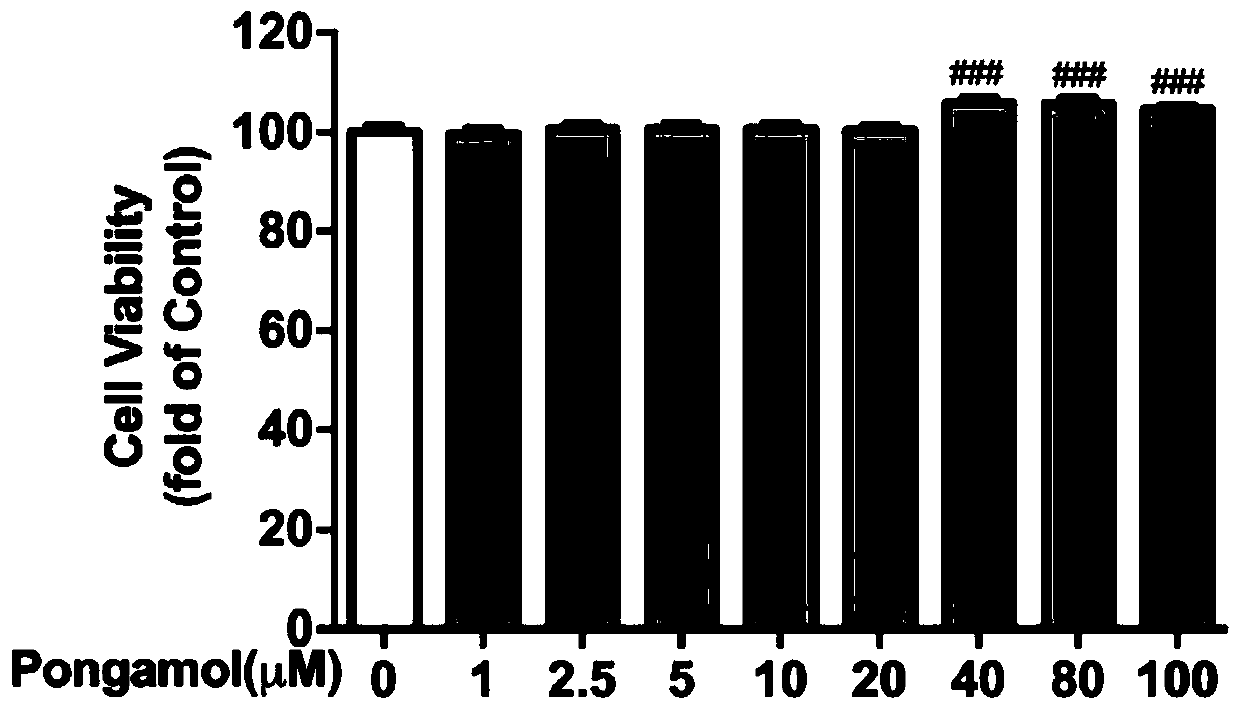
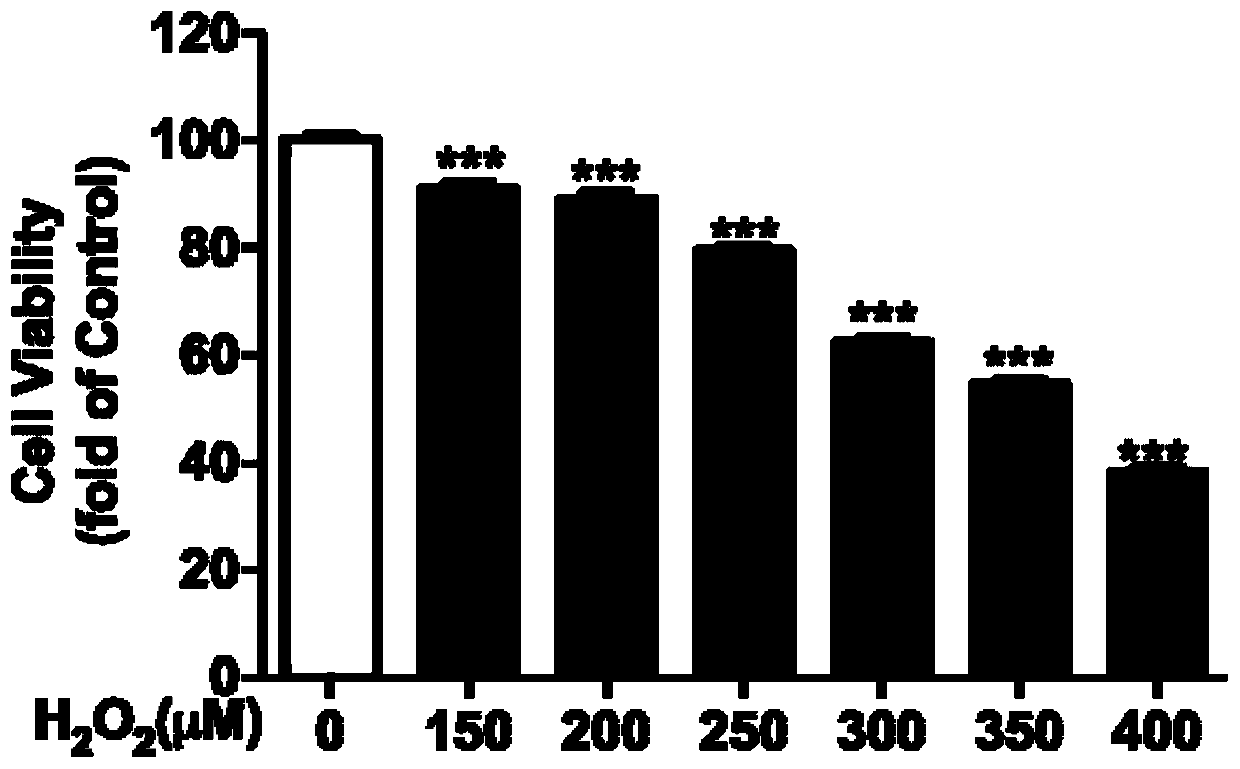
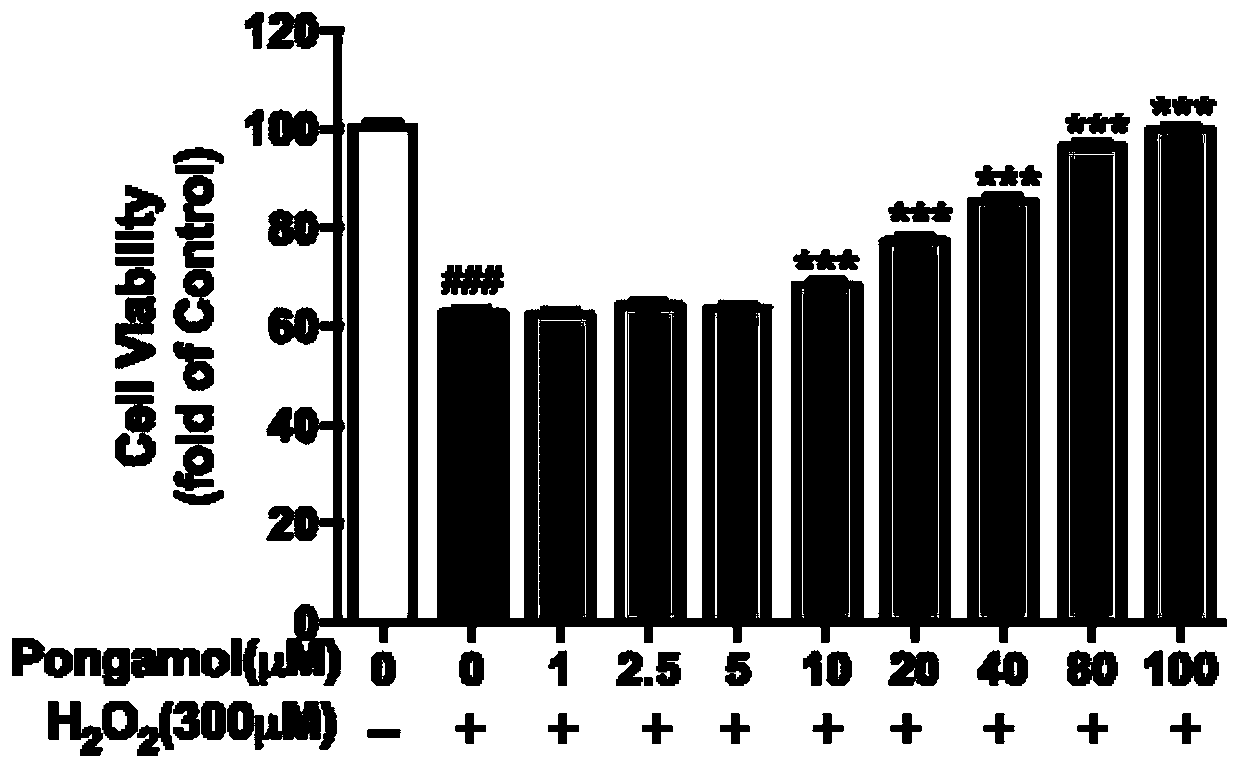
Preparation method and application of pongamol
A technology of pangetin and reaction, applied in antipyretics, nervous system diseases, organic chemistry, etc., can solve the problems of expensive starting materials, cumbersome post-processing, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] (1) Synthesis of 6,7-dihydro-4(5H)-benzofuranone
[0091] Add 1,3-cyclohexanedione (10.0g, 89.18mmol) to a 250mL round bottom flask, dissolve it in 60mL of water, add 20% sodium hydroxide solution 35mL under ice-cooling, then add KI (2.9g, 17.4mmol) , and finally 16 mL of 40% chloroacetaldehyde aqueous solution was slowly added dropwise. Add concentrated hydrochloric acid with a mass fraction of 37.5% to adjust the pH to about 4, add 100mL of water, extract with petroleum ether (100mL×4), combine the organic phases, wash with saturated brine (100mL×3), and finally wash with anhydrous sodium sulfate After drying, the solvent was evaporated to dryness under reduced pressure to obtain 10.0 g of light yellow liquid 7, namely 6,7-dihydro-4(5H)-benzofuranone, with a yield of 82%. 1 H NMR (400MHz, CDCl 3 )δ: 7.32(d, J=2.0Hz, 1H), 6.67(d, J=2.0Hz, 1H), 2.90(m, 2H), 2.52(m, 2H), 2.21(m, 2H).
[0092] (2) Synthesis of 5-acetyl-6,7-dihydro-4(5H)-benzofuranone
[0093] Add 7 (7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com