A F127-induced three-dimensional porous feni-nc bifunctional electrocatalyst and its preparation method
A F127, three-dimensional porous technology, applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of high cost, difficult industrialization, complicated preparation methods, etc., and achieve low toxicity, low price, The effect of preparation process safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
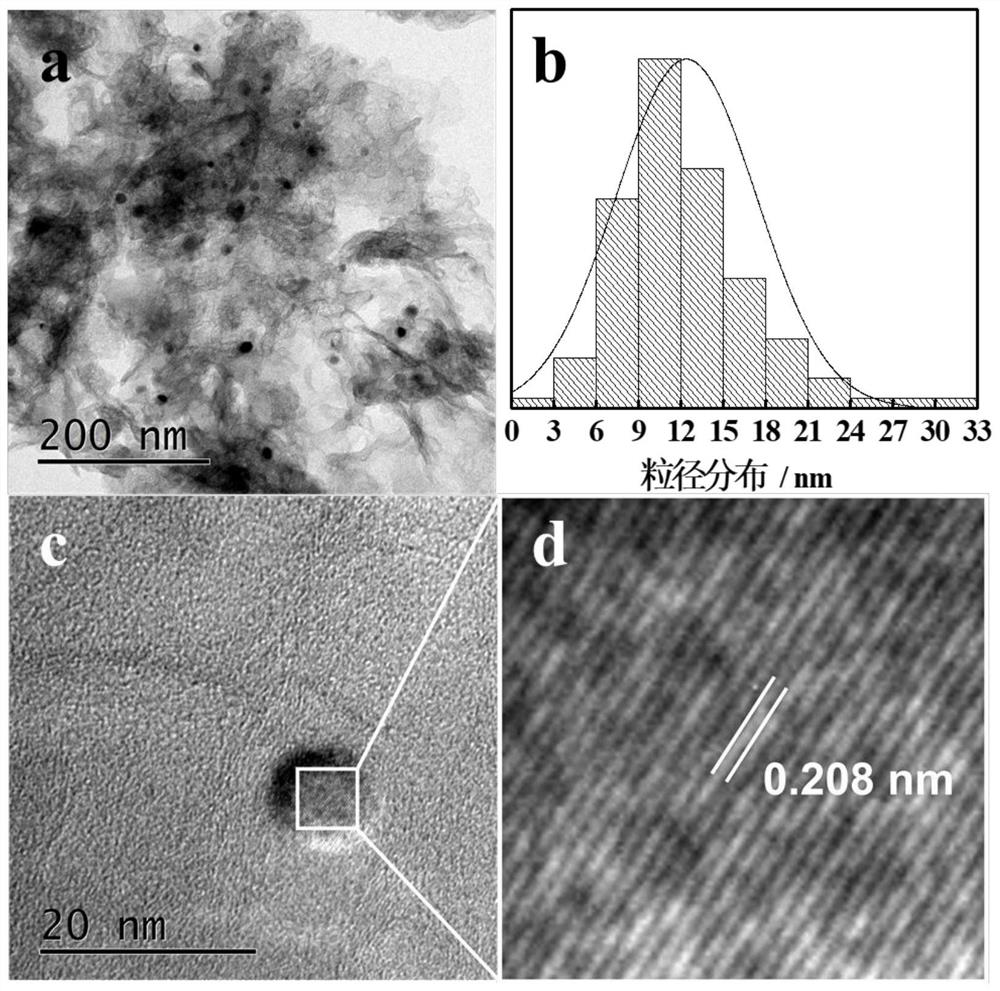
[0042] Example 1: Fe 1 Ni 1 -NC 4 -F127 3 -800-2 (Fe 1 Ni 1 Refers to the addition of Fe(NO 3 ) 3 9H 2 O and NiCl 2 ·6H 2 The molar ratio of O is 1:1, NC 4 Indicates gC produced by calcination of melamine 3 N 4 , F127 3 Indicates that F127 was added during the preparation process, NC 4 -F127 3 Refers to the addition of gC during the preparation process 3 N 4 The mass ratio to F127 is 4:3, 800-2 means the pyrolysis temperature is 800°C, and the pyrolysis time is 2h)
[0043] Weigh 8g of melamine and grind it evenly, place it in a tube furnace, and put it under N 2 Under atmosphere at 5°C min -1 The temperature was programmed to rise to 550°C, and the constant temperature was reacted for 4 hours, and gC was obtained after natural cooling. 3 N 4 sample. Dissolve 300mg F127 in 20mL deionized water, add 400mg gC 3 N 4 , ultrasonically disperse for 1h, add 60.6mg Fe(NO 3 ) 3 9H 2 O and 35.6 mg NiCl 2 ·6H 2 O, stirred at 80°C for 10h, and dried at 80°C for...
Embodiment 2
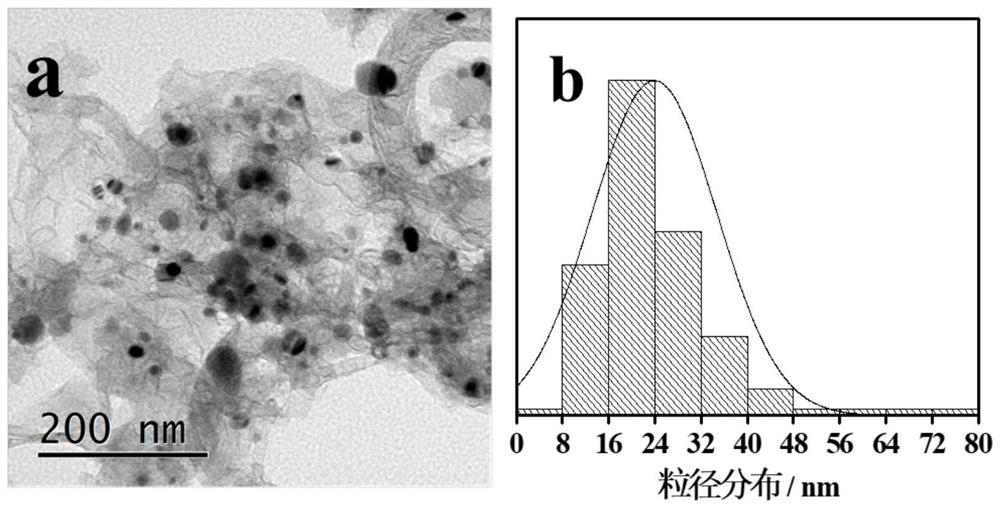
[0044] Example 2: Fe 1 Ni 1 -NC 4 -F127 3 -700-2 (Fe 1 Ni 1 Refers to the addition of Fe(NO 3 ) 3 9H 2 O and NiCl 2 ·6H 2 The molar ratio of O is 1:1, NC 4 Indicates gC produced by calcination of melamine 3 N 4 , F127 3 Indicates that F127 was added during the preparation process, NC 4 -F127 3 Refers to the addition of gC during the preparation process 3 N 4 The mass ratio to F127 is 4:3, 700-2 means the pyrolysis temperature is 700°C, and the pyrolysis time is 2h)
[0045] Weigh 8g of melamine and grind it evenly, place it in a tube furnace, and put it under N 2 Under atmosphere at 5°C min -1 The temperature was programmed to rise to 550°C, and the constant temperature was reacted for 4 hours, and gC was obtained after natural cooling. 3 N 4 sample. Dissolve 300mg F127 in 20mL deionized water, add 400mg gC 3 N 4 , ultrasonically disperse for 1h, add 60.6mg Fe(NO 3 ) 3 9H 2 O and 35.6 mg NiCl 2 ·6H 2 O, stirred at 80°C for 10h, and dried at 80°C for...
Embodiment 3
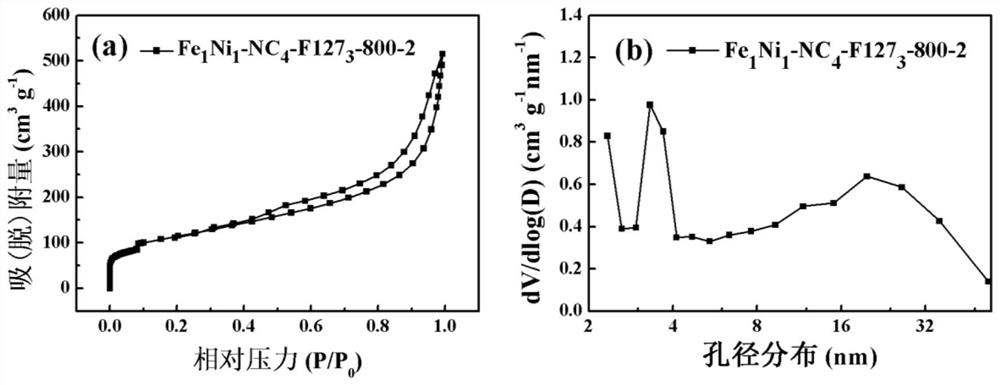
[0046] Example 3: Fe 1 Ni 1 -NC 4 -F127 3 -900-2 (Fe 1 Ni 1Refers to the addition of Fe(NO 3 ) 3 9H 2 O and NiCl 2 ·6H 2 The molar ratio of O is 1:1, NC 4 Indicates gC produced by calcination of melamine 3 N 4 , F127 3 Indicates that F127 was added during the preparation process, NC 4 -F127 3 Refers to the addition of gC during the preparation process 3 N 4 The mass ratio to F127 is 4:3, 900-2 means the pyrolysis temperature is 900°C, and the pyrolysis time is 2h)
[0047] Weigh 8g of melamine and grind it evenly, place it in a tube furnace, and put it under N 2 Under atmosphere at 5°C min -1 The temperature was programmed to rise to 550°C, and the constant temperature was reacted for 4 hours, and gC was obtained after natural cooling. 3 N 4 sample. Dissolve 300mg F127 in 20mL deionized water, add 400mg gC 3 N 4 , ultrasonically disperse for 1h, add 60.6mg Fe(NO 3 ) 3 9H 2 O and 35.6 mg NiCl 2 ·6H 2 O, stirred at 80°C for 10h, and dried at 80°C for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com