Preparation method of N-(indol-N-formyl)-alpha-aminoamide derivatives
A technology of aminoamide and formyl, which is applied in the field of preparation of N--α-aminoamide derivatives, achieving the effect of mild conditions and excellent yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] In a dry two-necked reaction flask, 33 mg (0.2 mmol) of N-indolecarboxylic acid and 36 mg (0.24 mmol) of p-nitrobenzaldehyde were weighed, and 2 mL of solvent (dichloromethane:methanol=1:2) was injected after being protected by an argon balloon. , inject aniline 24mg and benzyl isocyanide 28mg subsequently, the reaction is stirred under ice-water bath for 24 hours, TLC detects that the reaction has been converted completely, and after adding silica gel to mix the sample, column chromatography separation (eluent is petroleum ether: ethyl acetate=5: 1) 74 mg of the product can be obtained with a yield of 73%. The reaction formula is as follows:
[0041]
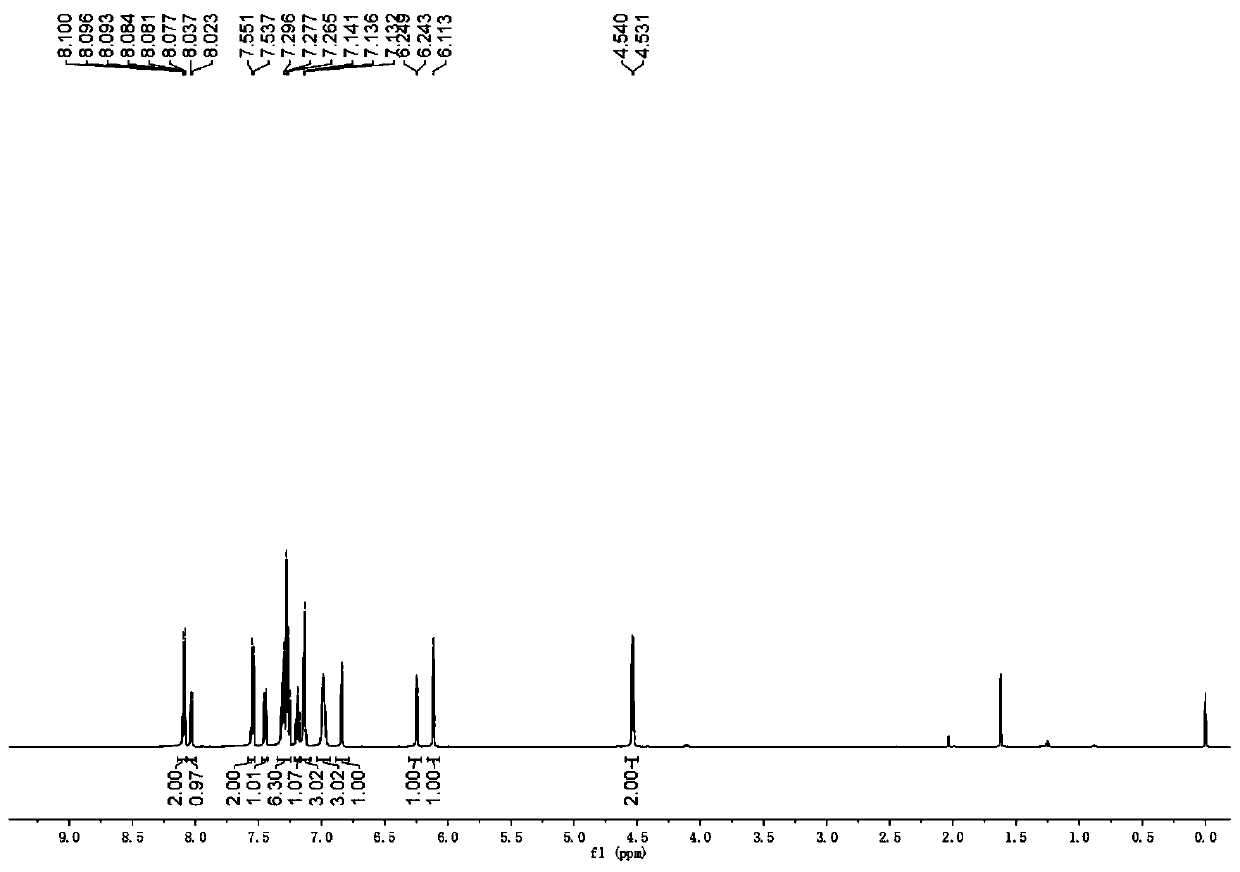
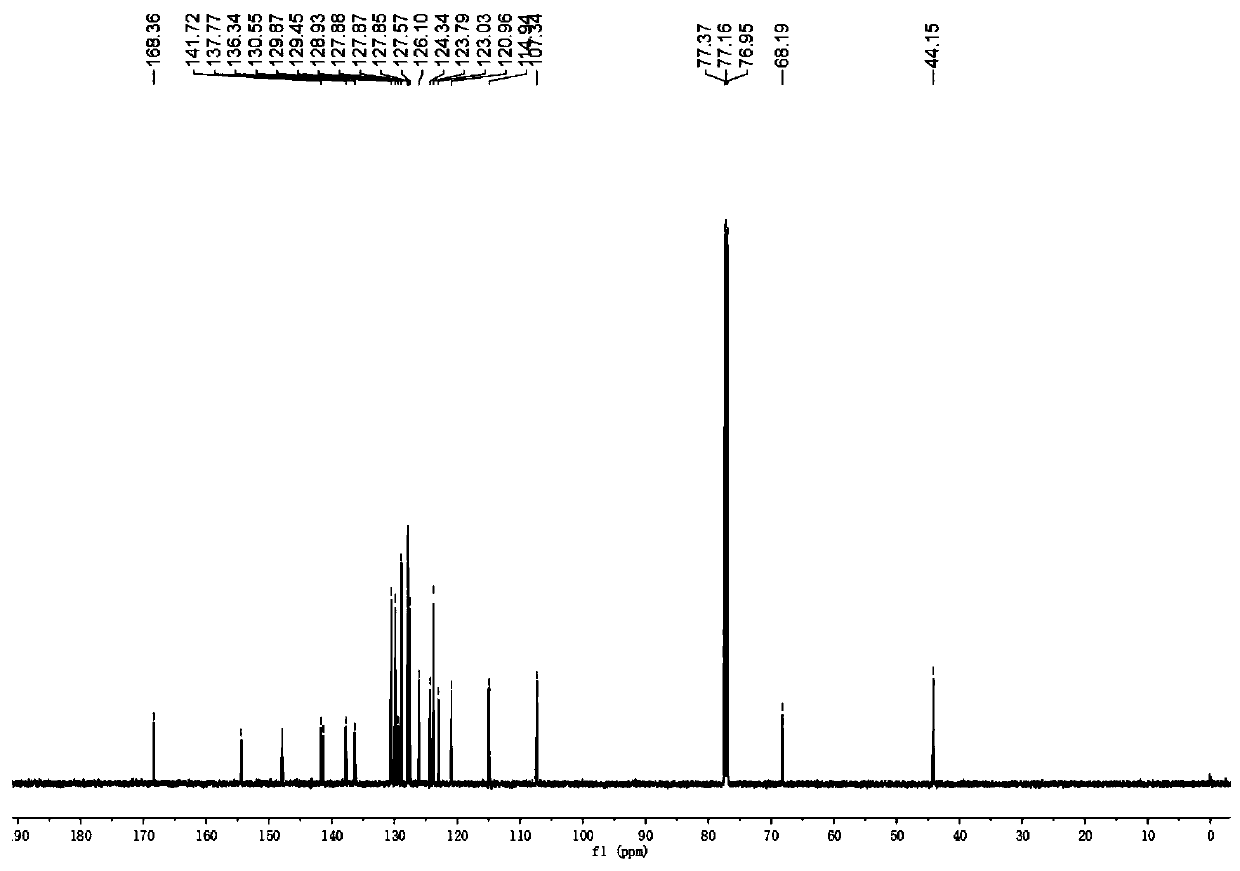
[0042] The physical properties and spectral data of the product are as follows: white solid, melting point 151–153 °C; 1 HNMR (600MHz, CDCl 3 )δ8.13–8.07(m,2H),8.03(d,J=8.4Hz,1H),7.54(d,J=8.4Hz,2H),7.45(d,J=7.8Hz,1H),7.35– 7.24 (m, 6H), 7.21–7.09 (m, 4H), 7.04–6.93 (m, 3H), 6.84 (d, J=3.6Hz, 1H), 6.25 (d, J=3.6Hz, ...
Embodiment 2
[0044]In a dry two-necked reaction flask, 33 mg (0.2 mmol) of N-indolecarboxylic acid and 26 mg (0.24 mmol) of benzaldehyde were weighed, and 2 mL of solvent (dichloromethane:methanol=1:2) was injected after being protected by an argon balloon, and then injected 24 mg of aniline and 28 mg of benzyl isocyanide were reacted in an ice-water bath and stirred for 24 hours. TLC detected that the reaction was completely converted. After adding silica gel and mixing the sample, column chromatography separation (eluent was petroleum ether: ethyl acetate = 5: 1) could be obtained. The product was obtained in 63 mg with a yield of 69%. The reaction formula is as follows:
[0045]
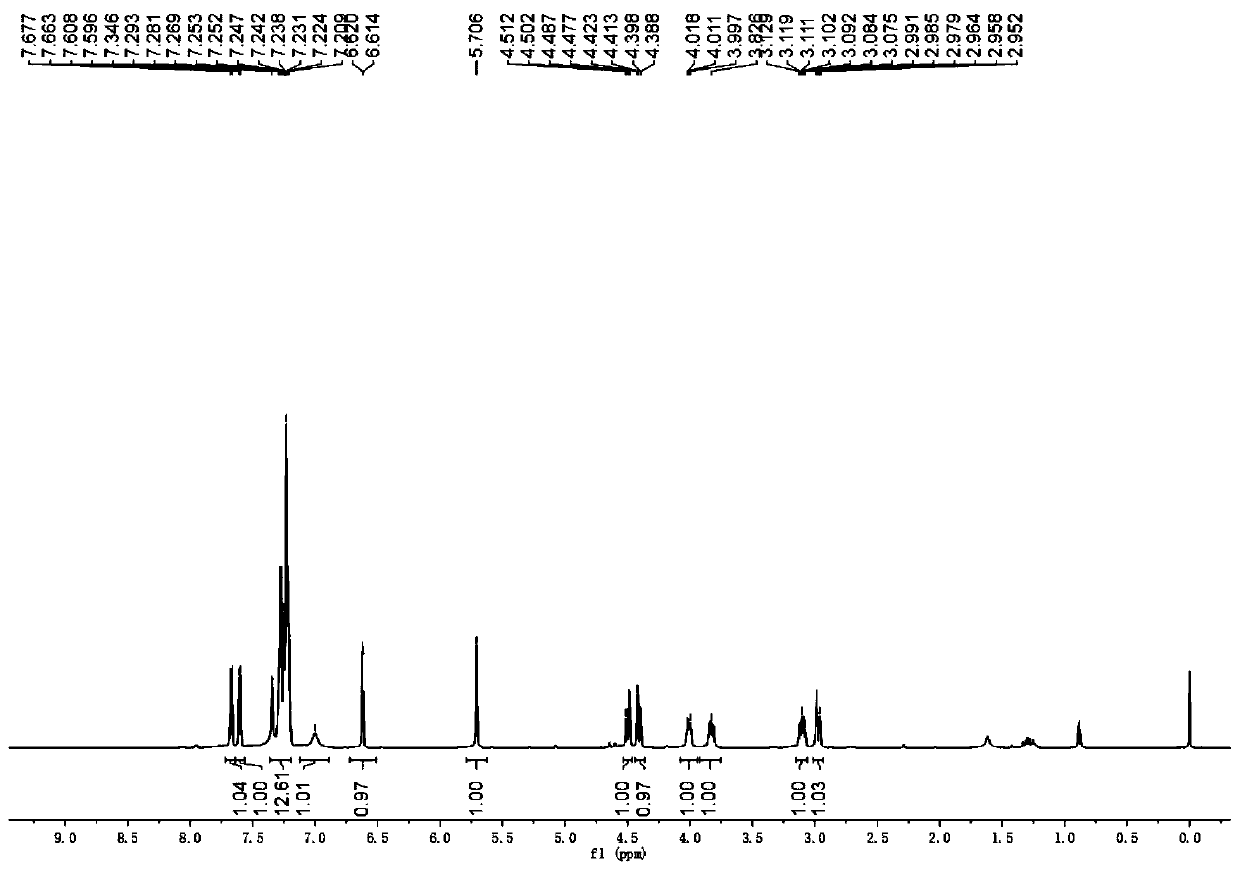
[0046] The physical properties and spectral data of the product are as follows: white solid, melting point 141–143 °C; 1 H NMR (600MHz, CDCl 3 )δ8.13-8.04(m,1H),7.43(d,J=7.8Hz,1H),7.40-7.36(m,2H),7.32-7.23(m,9H),7.18-7.15(m,1H) ,7.12–7.06(m,3H),7.06–7.00(m,2H),6.87(d,J=3.6Hz,1H),6.50(t,J=5.4Hz,1H),6.22(d...
Embodiment 3
[0048] In a dry two-necked reaction flask, 33 mg (0.2 mmol) of N-indolecarboxylic acid and 49 mg (0.24 mmol) of 3-bromo-4-fluorobenzaldehyde were weighed, and 2 mL of solvent (dichloromethane: methanol = 1:2), inject aniline 24mg and benzyl isocyanide 28mg subsequently, the reaction is stirred under ice-water bath for 24 hours, TLC detects that the reaction has been converted completely, and after adding silica gel to mix the sample, column chromatography separation (eluent is petroleum ether: ethyl acetate) Ester=5:1) 89 mg of product can be obtained, and the yield is 80%. The reaction formula is as follows:
[0049]
[0050] The physical properties and spectral data of the product are as follows: white solid, melting point 134–136 °C; 1 H NMR (400MHz, CDCl 3 )δ8.05(d,J=8.4Hz,1H),7.56(dd,J 1 =6.4Hz,J 2 =2.0Hz,1H),7.44(d,J=7.6Hz,1H),7.34-7.23(m,7H),7.21-7.08(m,4H),7.03-6.92(m,3H),6.83(d, J=3.6Hz, 1H), 6.73(t, J=6.0Hz, 1H), 6.22(d, J=3.6Hz, 1H), 6.00(s, 1H), 4.52(d, J=6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com