A kind of high-efficiency and fast-binding vegf antibody and its use
A technology of antibodies and antibody fragments, applied in anti-growth factor immunoglobulins, anti-animal/human immunoglobulins, instruments, etc., can solve the problems of little significance in early screening and achieve the effect of suppressing immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] To obtain high-titer VEGF antibody, the specific method is:
[0043] Step 1, mice immunization, using 6 to 8 weeks old female BALB / c mice as experimental animals (purchased from Shanghai Lingchang Biotechnology Co., Ltd.), VEGF-A antigen protein (purchased from Guangzhou Ruibo Biotechnology Co., Ltd. ).
[0044]Step 2. Dissolve the antigenic protein in normal saline, mix 50 μg of human with an equal volume of complete Freund’s adjuvant for the first immunization, and inject mice at 1 ml / mouse, subcutaneously in the abdomen at multiple points.
[0045] Step 3. Booster immunization was carried out after 2 weeks. For booster immunization, 25 μg of human VEGF protein was fully mixed with incomplete Freund’s adjuvant to form an emulsion, and injected into the abdominal cavity of mice at an injection volume of 0.5 ml / mouse, and boosted immunization 3 times.
[0046] Step 4. Three days after the last immunization, the venous blood of the mice was taken and the serum was separ...
Embodiment 2
[0051] Coat human VEGF (purchased from Guangzhou Ruiboao Biotechnology Co., Ltd.) on a 96-well high-adsorption enzyme plate with carbonate buffer solution, the coating volume is 100 μ L per well, and the buffer solution is washed 3 times; The blocking buffer was blocked and incubated at room temperature for 1 hour. The blocking volume was 250 μL / well. After the incubation was completed, the buffer was washed 3 times, and 100 μL of supernatant samples (A1-A85) and positive serum were added to wells 1-90 respectively. (control, CK1-5), incubate at room temperature for 1 hour, wash buffer 3 times after incubation, add 100 μL anti-mouse IgG antibody diluted in 1% BSA buffer at a ratio of 1 / 10000 to each well, the anti-mouse IgG antibody Mouse IgG antibody was labeled with horseradish peroxidase and incubated at room temperature for 1 hour. After incubation, the buffer was washed 3 times, and 100 μL of colorimetric substrate 3,3',5,5'-tetramethylbenzidine was added to each well. Af...
Embodiment 3
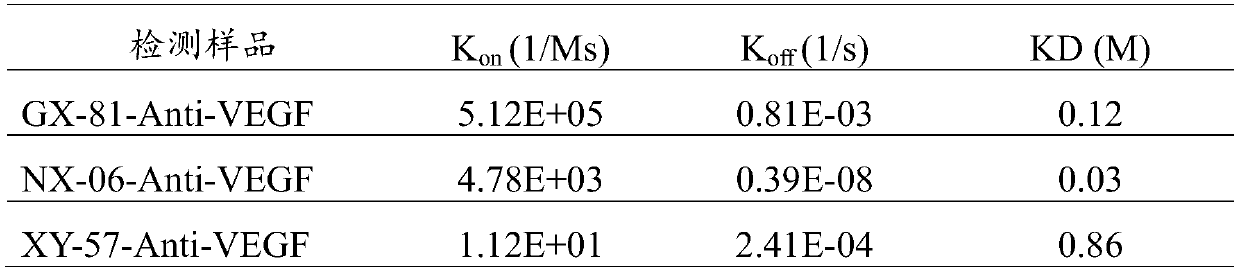
[0053] In Example 2, a total of three clones GX-81, NX-06, and XY-57 with strong antigen-binding activity were obtained, and the screened clones with both strong antigen-binding activity and antigen-neutralizing activity were subjected to nucleotide Sequence determination, briefly, the first strand of cDNA was synthesized after extracting the cellular mRNA, the first strand of cDNA generated by reverse transcription was used in the subsequent PCR reaction, the target band obtained by PCR amplification was cloned into the pGEM-T vector, and picked Single clones were subjected to DNA sequencing. Sequencing was performed by Nanjing GenScript Biotechnology Co., Ltd.
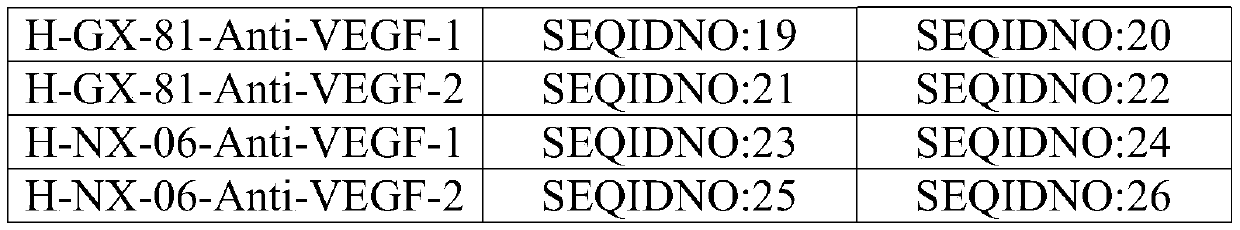
[0054] The antibody light chain variable region and the antibody heavy chain variable region are obtained by PCR amplification, and the complementarity determining region sequence can be obtained after excluding the framework region sequence:
[0055] The amino acid sequence of the three complementarity determining ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com