Bi-porous covalent organic framework material linked by imine bonds and preparation and application thereof
A technology of covalent organic skeleton and imine bond, applied in the field of porous materials, can solve the problems of low photocatalytic reduction of carbon dioxide, in the primary stage, etc., and achieve the effect of good carbon dioxide activation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
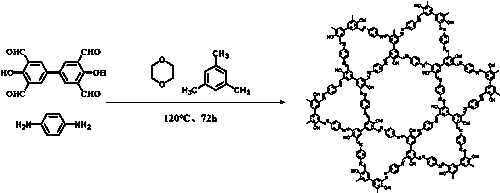
[0022] The method for preparing the above-mentioned covalent organic framework material linked by imine bonds is characterized in that it comprises the following steps:
[0023] (1) Add 3,3',5,5'-tetraformyl-4,4'-biphenyldiol, p-phenylenediamine, 1,4-dioxane and mesitylene to the vacuum tube in sequence . Sonicate at room temperature for 1 hour, add acetic acid after sonication, and perform three liquid nitrogen freezing and degassing operations to achieve a vacuum and oxygen-free environment.
[0024] (2) Put the vacuum tube processed in step (1) into an oven for reaction, raise the temperature of the oven to 120° C., and react at this temperature for 72 hours. The oven temperature was lowered to room temperature, and the crude product was obtained by filtration.
[0025] (3) Wash the obtained crude product with tetrahydrofuran until the filtrate is colorless, perform solvent exchange with acetone, and vacuum-dry at 100° C. for 24 hours to obtain a dark reddish-brown solid ...
Embodiment 1
[0031] 0.0447g (0.15mmol) 3,3',5,5'-tetraformyl-4,4'-diphenol, 0.0324g (0.3mmol) p-phenylenediamine, 1.5mL 1,4-diox Hexacyclone and 1.5 mL of 1,3,5-trimethylbenzene were sequentially added to the vacuum tube. Sonicate at room temperature for 1 hour. After sonication, use a pipette gun to accurately add 0.5 mL of acetic acid with a substance concentration of 1 M, and perform three liquid nitrogen freezing and degassing operations. The vacuum tube in a sealed state was put into an oven for reaction, and the temperature of the oven was raised to 120° C., and the reaction was carried out at this temperature for 72 hours. After the reaction, the temperature of the oven was lowered to room temperature, filtered, washed with tetrahydrofuran until the solution was clear and transparent, solvent exchanged with acetone, and vacuum-dried at 100°C for 24 hours to obtain 0.065 g of a dual-porous covalent organic framework material linked by imine bonds .
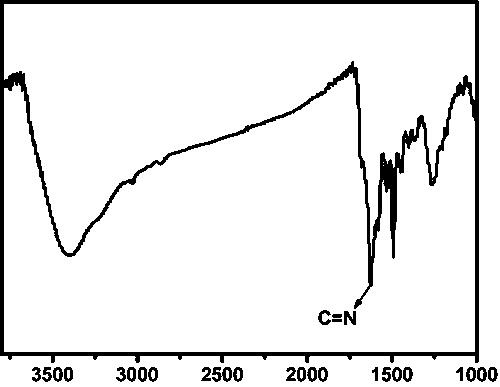
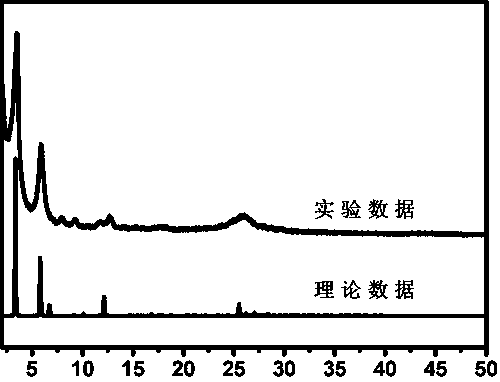
[0032] The infrared spectrum of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com