A kind of isopentenyl stilbene in two-color jackfruit and its use in the preparation of medicines for treating inflammatory diseases
A technology for isopentenyl stilbene and inflammatory diseases, which is applied in the application field of isopentenyl stilbene in the preparation of medicines for the treatment of inflammatory diseases, and can solve the problems of limited effect of PMNs respiratory burst inhibition and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
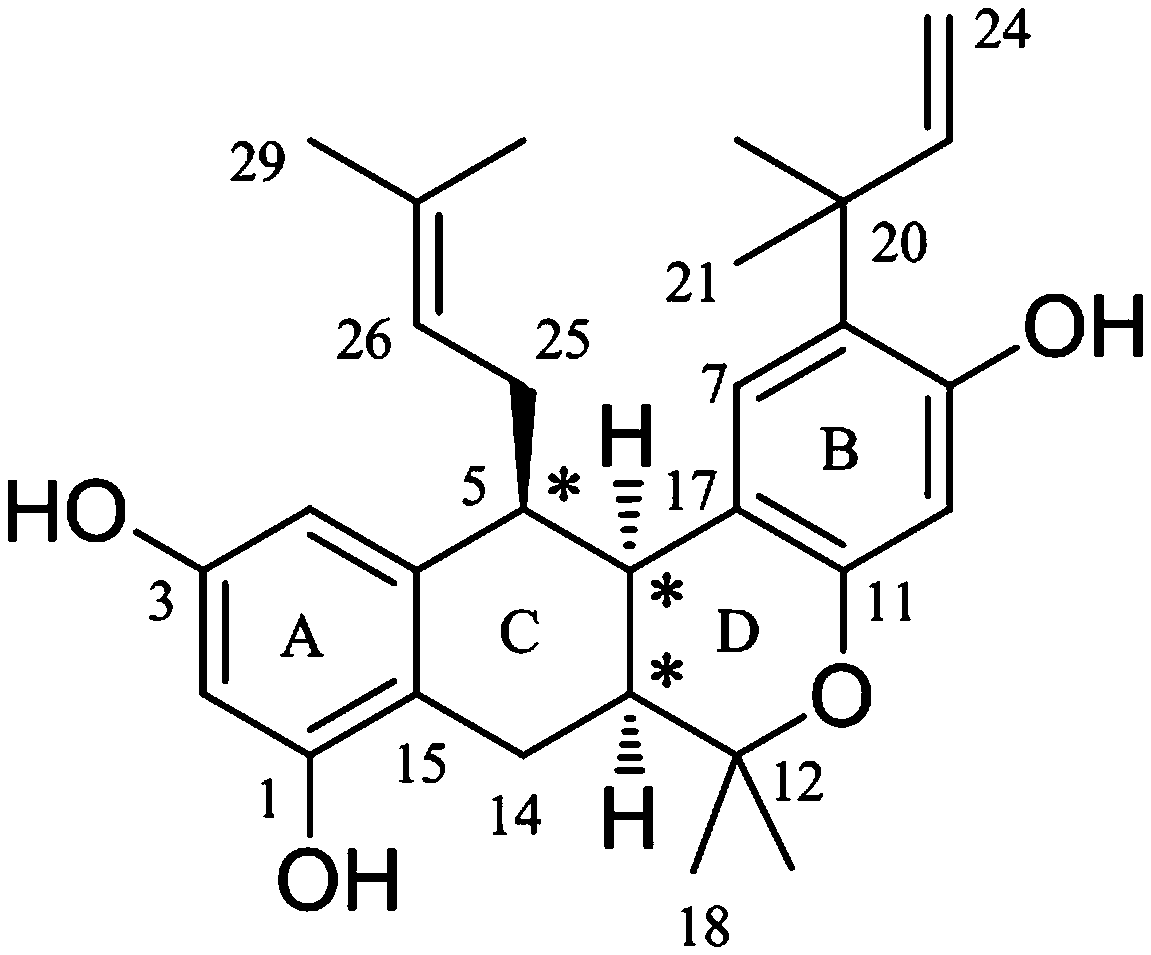
[0020] Example 1 Preparation of isopentenyl stilbene (±)-dichrome pirochol D in dichroma jackfruit
[0021] Take 13.9 Kg of jackfruit root, extract by leakage with 95% ethanol, and concentrate the extract under reduced pressure to obtain 1.3 Kg of extract. The extract was suspended in 1 L of water, extracted successively with petroleum ether, chloroform, ethyl acetate and n-butanol (volume ratio 2:1), and concentrated to dryness respectively. Take 118.9 g of the extract from the chloroform extraction part, mix the sample with HP-20 macroporous adsorption resin (weight ratio 1:1), put it on HP-20 type macroporous adsorption resin column (column specification: 10*45 cm), and mix with ethanol-water (0~95%) gradient elution to obtain 6 fractions Frs. H1-H6. 50% ethanol eluted fraction Fr. H4 (44.8 g) was subjected to ODS column chromatography (column size: 4*22 cm), MeOH -H 2 O (volume ratio 6:4, 7:3, 8:2, 9:1, 10:0) gradient elution to obtain 15 fractions Frs. H4O1-H4O15. Frac...
Embodiment 2
[0022] Example 2 Structural identification of isopentenyl stilbene (±)-dichrome pirolenol D in dichroma jackfruit
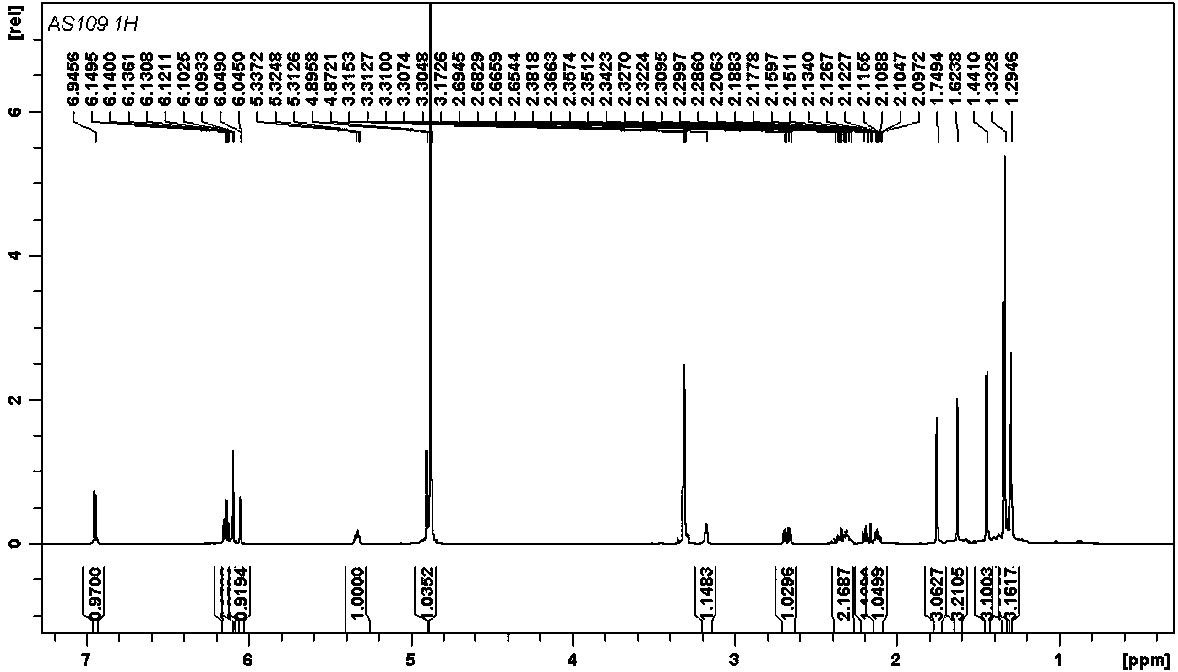
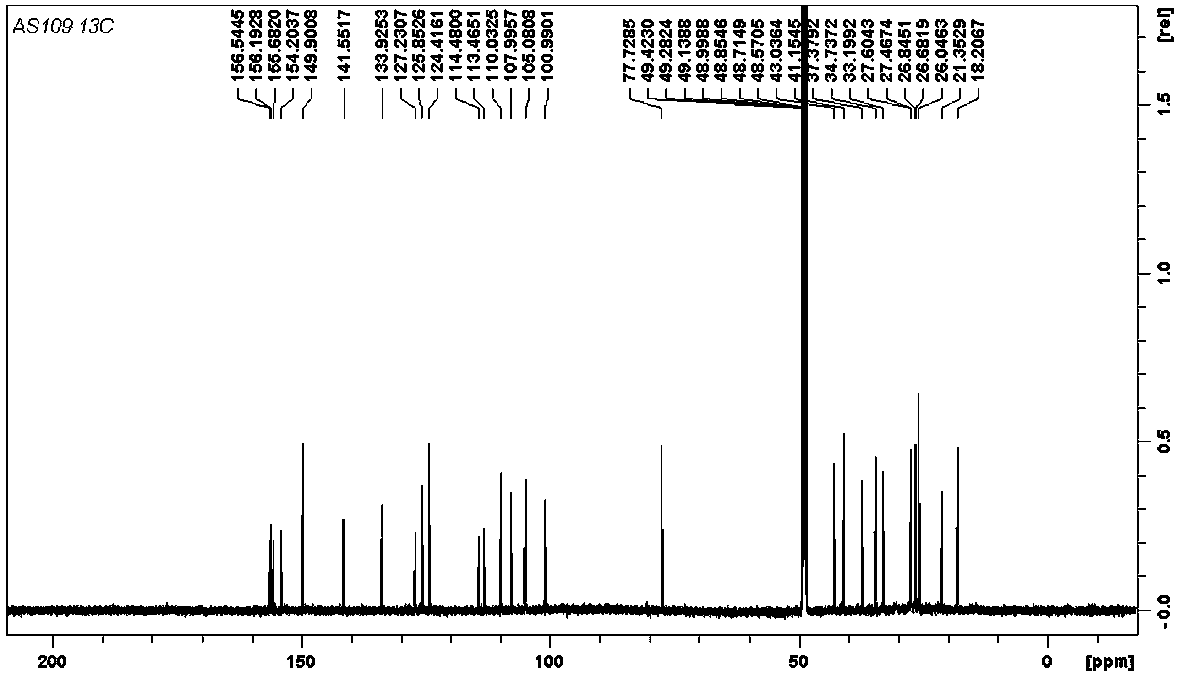
[0023] (±)-Dichroic pirophenol D, a yellow amorphous powder (methanol). HR-ESI-MS gives quasi-molecular ion peaks m / z 447.2526 ([M-H] - , Calculated value: 447.2541), determine its molecular formula as C 29 h 36 o 4 . (±)-Dichroic pirochol D 1 H NMR spectrum (600 MHz, methanol- d 4 ) is very similar to the known bis-prenyl substituted stilbene hypargystilbene D. Compared with hypargystilbene D, (±)-dichromophorol D has an additional set of proton signals of 3-methyl-2-butenyl isopentenyl. This suggests that (±)-dichromobolol D is a triisopentenyl substituted stilbene derivative. In the HMBC spectrum of (±)-dichromol D, we observed H 2 -25 ( δ H 2.33) and C-5 ( δ C 43.0), C-6 ( δ C 33.2), C-16 ( δ C 141.6), and H-26 ( δ H 5.32) Correlation with C-5 ( Figure 7 ). This indicates that a 3-methyl-2-butenyl type isopentenyl substitut...
Embodiment 3
[0026] Example 3 (±)-Cytotoxicity evaluation experiment of dichromol D to rat PMNs
[0027] Rat PMNs were isolated and purified using the following experimental procedures. Take clean SD rats (Jiangxi University of Traditional Chinese Medicine Experimental Animal Center, animal qualification certificate number: JZDW2011304), take 9 mL of blood from the orbit, and drop it vertically into a glass centrifuge tube that has been anticoagulated with 1 mL of 1% heparin sodium. Add 4.5% dextran T-500 saline solution at a ratio of 5:1, mix well, and let stand at 4°C for about 1 hour. Take the supernatant, add it to the centrifuge tube pre-filled with lymphocyte separation medium at a ratio of 3:1, 800 rpm (275 g ) Centrifuge for 15 minutes, take out the centrifuge tube, the tube is divided into three layers, the upper layer is light yellow serum, the middle white misty area is monocytes and lymphocytes, and the lower layer is PMNs that settle to the bottom of the tube. Discard the su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com