Preparation method and application of triazine compound with polyvinyl chloride light stabilization function
A technology of polyvinyl chloride and triazines, which is applied in the field of preparation of triazines, can solve the problems of different triggering conditions, loss of use value, decline in PVC mechanical properties, etc., and achieve good light stabilization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
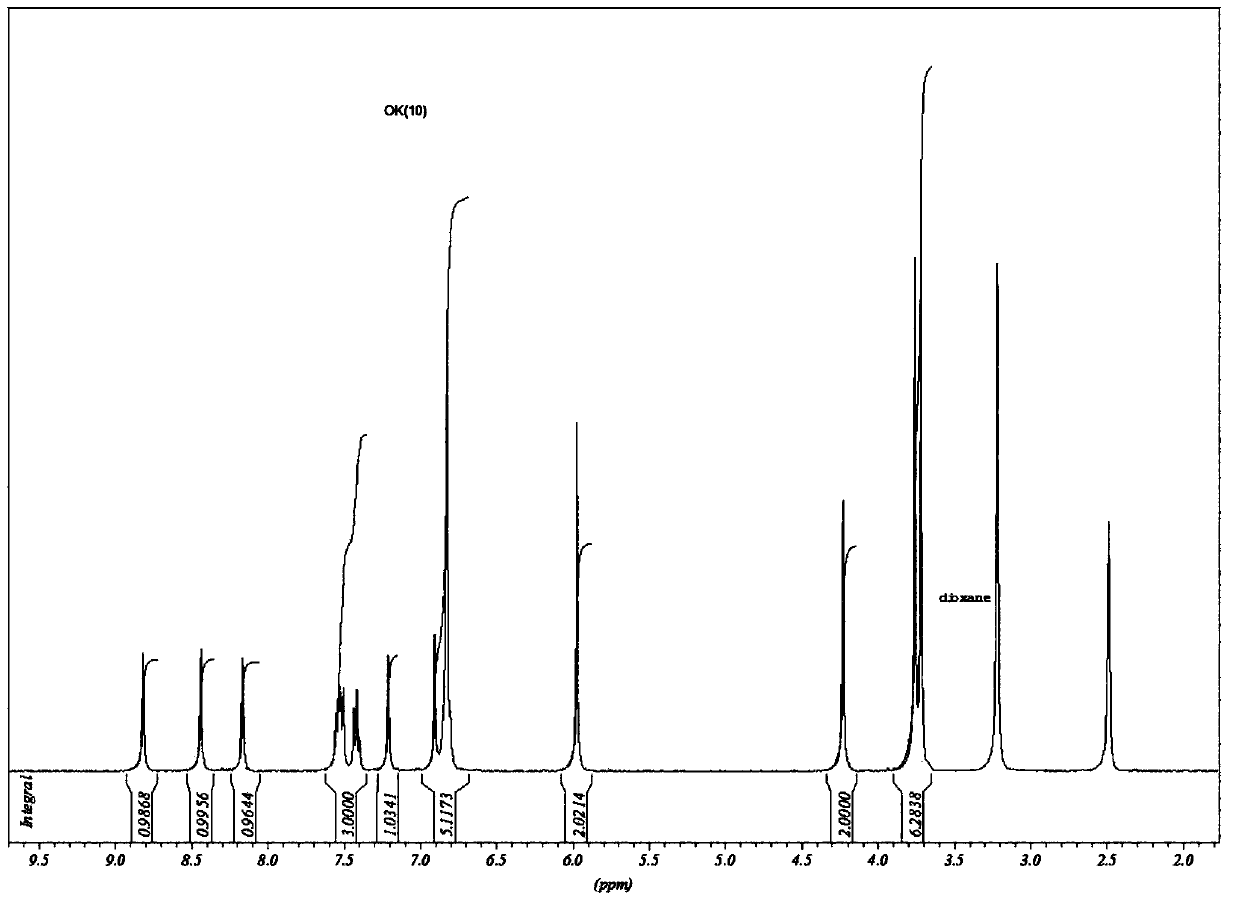
Image
Examples
Embodiment 1
[0021]
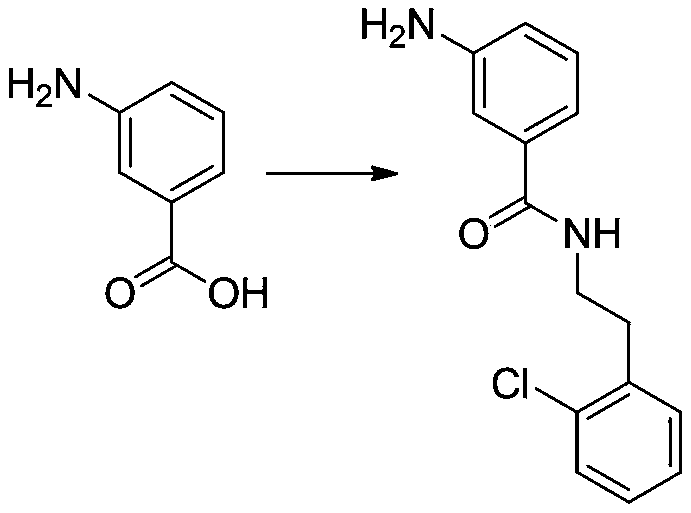
[0022] Under nitrogen protection, add 14g (0.1mol) of m-aminobenzoic acid (0.1mol) and 200mL of N,N-dimethylformamide into a 500mL four-neck flask, stir at room temperature and dissolve completely, then add 25g (0.15mol) of N,N'-carbonyldiimidazole ), it can be seen that the solution is turbid; start heating, slowly heat up to 60°C, keep stirring at this temperature for 1.5h, stop heating, and continue stirring; the system is cooled to room temperature 25°C, and 16g of o-chlorophenethylamine is added dropwise through the dropping funnel (0.1mol) of N,N-dimethylformamide 100mL, stirred and reacted at room temperature for 5h after the dropwise addition, TLC monitored the complete reaction of the raw materials, added 300mL of water to the reaction solution, and adjusted the pH of the reaction solution with dilute hydrochloric acid 8 to 9, filter the reaction solution, extract the filtrate with 100 mL of dichloromethane for several times, combine the organic phases, dry...
Embodiment 2
[0024] Under nitrogen protection, add 14g (0.1mol) of m-aminobenzoic acid (0.1mol) and 200mL of N,N-dimethylformamide into a 500mL four-neck flask, stir at room temperature and dissolve completely, then add 16g (0.1mol) of N,N'-carbonyldiimidazole ), it can be seen that the solution is turbid; start heating, slowly heat up to 80°C, keep the temperature and continue stirring for 3.5h, stop heating, and continue stirring; the system is cooled to room temperature 25°C, and 16g of o-chlorophenethylamine is added dropwise through the dropping funnel (0.1mol) of N,N-dimethylformamide 100mL, after the dropwise addition, stir and react at room temperature for 7h, add 300mL of water to the reaction solution, adjust the pH of the reaction solution to 8-9 with dilute hydrochloric acid, filter The reaction solution and the filtrate were extracted several times with 100 mL of dichloromethane, the organic phases were combined, dried over anhydrous magnesium sulfate, concentrated, and then se...
Embodiment 3
[0026]
[0027] Add 27.5 (0.1 mol) of 3-amino-N-(2 chlorophenethyl) benzamide, 7 g (0.1 mol) of sodium ethoxide, 43 g (0.3 mol) of methyl iodide and 300 mL of ethanol in the reaction flask, and the reaction temperature is set at React at -10°C for 5 hours, add 200 mL of distilled water to the reaction solution, distill off 200 mL of solvent ethanol under reduced pressure under vacuum, then extract the reaction solution with 100 mL of ethyl acetate three times, combine the organic phases, and undergo saturated chlorination. Wash twice with 100 mL of sodium solution, separate the organic phase, dry the obtained organic phase with anhydrous magnesium sulfate, concentrate and purify through silica gel column chromatography to obtain methoxy-3-amino-N-(2-chlorophenethyl ) benzyl imine 22.9g; MS (ESI) m / z: 289.7 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com