Reagent for cell infection by viruses and application of reagent
A virus infection and reagent technology, applied in the field of genetic engineering, can solve the problems of poor infection efficiency and complicated operation of T cells, and achieve the effect that the killing ability is not affected.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Expansion of primary T cells
[0029] Adopt the company's previous patent (application number 201810090357.1) amplification technology. It's as simple as:
[0030] 1. Activate the culture bottle: add the activator to the culture bottle until it covers the bottom of the bottle. 2 Add 10mL of activator to the cell culture flask to fill the bottom of the flask, and incubate at 4°C for 12 hours to obtain an activated culture flask. The activator is prepared by dissolving CD3 monoclonal antibody and IFN-γ in PBS buffer solution (pH 7.35-7.45). The concentration of CD3 monoclonal antibody was 50 μg / mL, and the concentration of IFN-γ was 2000 U / mL;
[0031] 2. Preparation of activation medium: Add 5mL of autologous plasma and 1mL of CIK cell activator to 44mL of basal medium to obtain an activation medium, wherein the CIK cell activator consists of IL-2, IL-1α and IFN-γ It is prepared by dissolving in PBS buffer solution, the concentration of IL-2 is 50000U / mL, th...
Embodiment 2
[0033] Example 2, the process of lentivirus production carrying CAR gene sequence
[0034] 1. 293T cell preparation:
[0035] Take out a frozen 293T cell (purchased from ATCC) from liquid nitrogen and quickly put it in a 37°C water bath until the ice cube disappears, add it dropwise to a 15ml centrifuge tube containing 5ml preheated medium, centrifuge at 1200rpm for 3min, discard Clear, resuspend the cells in 293T medium (10% FBS + 1mM sodium pyruvate + 2mM glutamine + 1% non-essential amino acids + DMEM) and inoculate them into 150mm culture dishes at 37°C, 5% CO 2 Cultivate in saturated humidity.
[0036] During the culture process, when the confluence of the cells reaches more than 90%, carry out subculture, discard the old medium, add 5ml sterilized PBS solution, shake gently, discard the PBS solution after washing the cells, and add 2ml 0.25% Trypsin-EDTA Digestion solution, digest for 1-2min until the cells are completely digested. Serum-containing medium was added to...
Embodiment 3
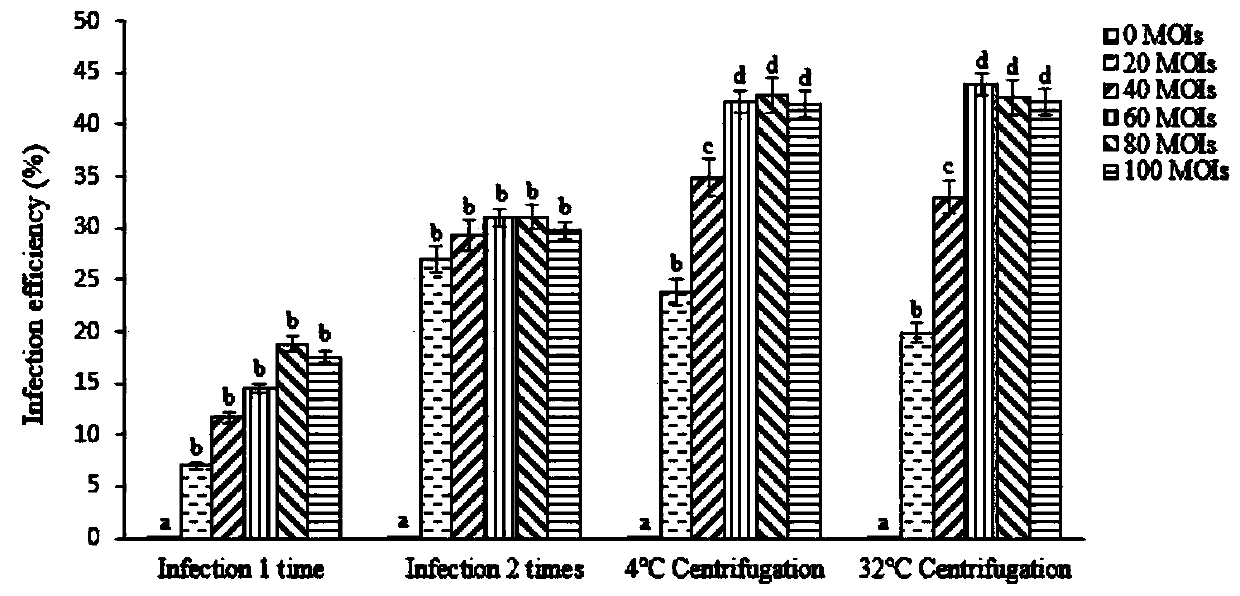
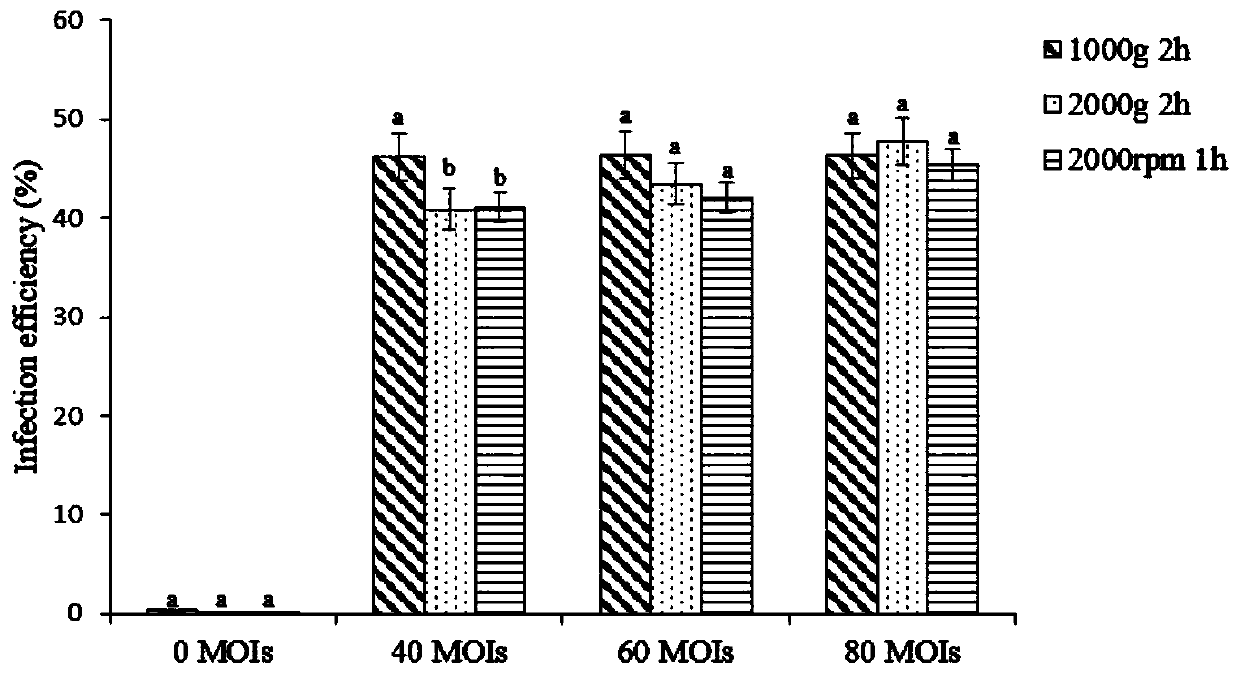
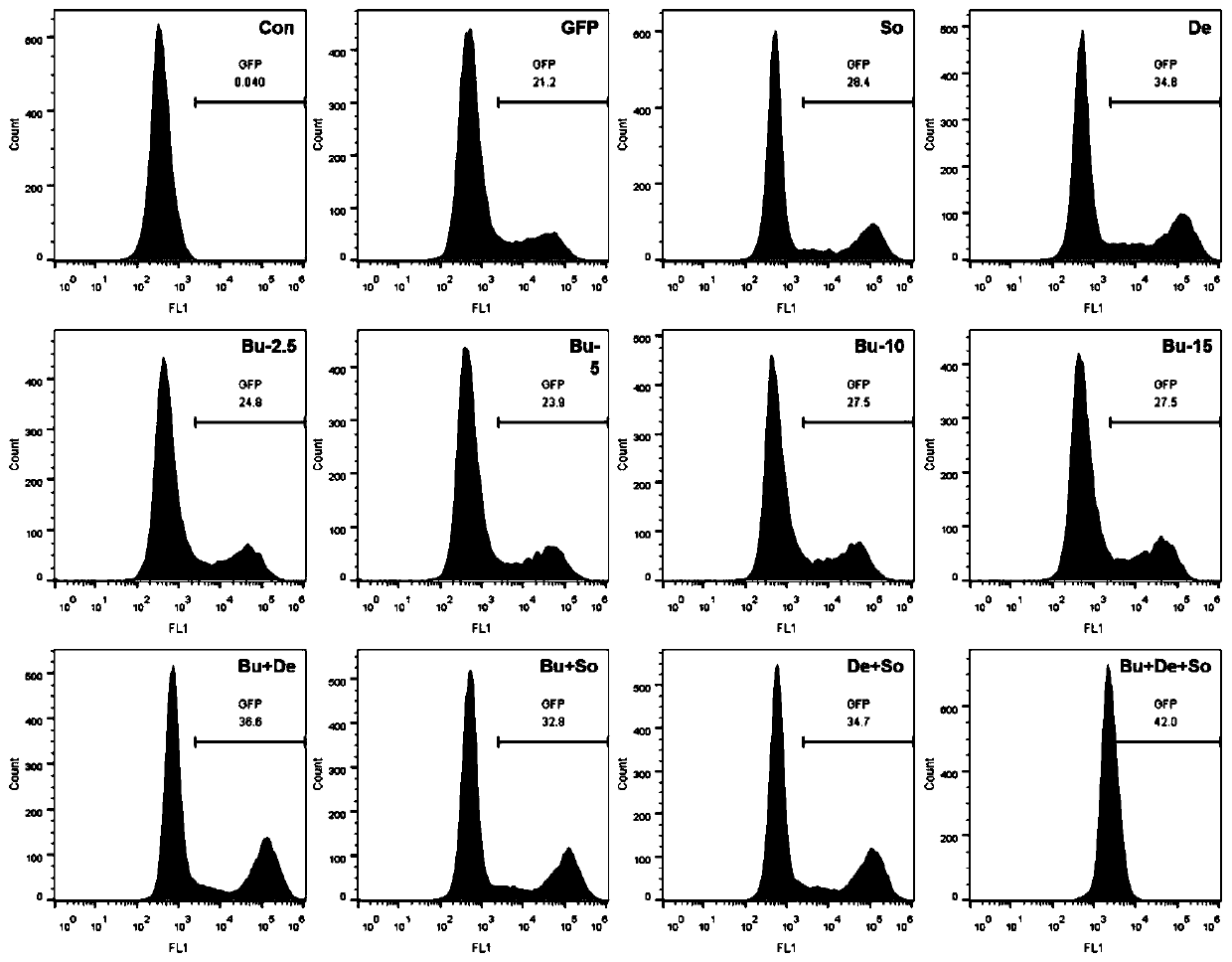
[0046] The infection efficiency of the EGFP virus of embodiment 3 different multiplicity of infection to primary generation T cell
[0047] The obtained high-titer lentiviral particles carrying the EGFP green fluorescent protein reporter gene were used to infect the obtained primary T cells, and the infection efficiency of different infection methods and different virus multiplicity on the primary T cells was explored.
[0048] The infection method is selected as follows: (1) directly add the virus into the T cell culture system for one infection; (2) directly add the virus into the T cell culture system for two infections, that is, replace the culture medium after the first infection, and then carry out one infection. (3) Centrifuge at 1000g at 4°C for 1h; (4) Centrifuge at 1000g at 32°C for 1h.
[0049] Multiplicity of Virus Infection We chose MOIs from 0-100 for infection, namely 0, 20, 40, 60, 80 and 100 MOIs. All groups were added 8 μg / ml polybrene as auxiliary infectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com