Conductive polymer coated Prussian blue sodium ion battery positive electrode material and preparation method thereof
A conductive polymer, sodium-ion battery technology, applied in the fields of energy materials and electrochemistry, can solve problems such as poor cycle performance and poor rate performance, achieve good rate performance and cycle stability, and be easy to large-scale industrial production. , the effect of improving conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
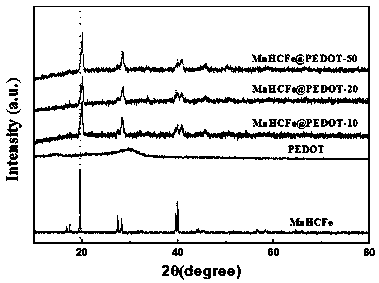
[0050] In this embodiment, the Prussian blue analog MnHCFe is prepared by the same method as that of Comparative Example 1, and the conductive polymer-coated Prussian blue sodium-ion battery cathode material is obtained by in-situ polymerization of the conductive polymer monomer on the surface. The specific steps are:
[0051] 1) Add 200 mg of MnHCFe and 10 μL of EDOT in Comparative Example 1 into 40 mL of deionized water, ultrasonically disperse and stir to obtain cloudy liquid C, and keep at 25 °C;
[0052] 2) Dissolve 20 mg ammonium persulfate in 10 mL deionized water to form solution D;
[0053] 3) Add solution D to turbid solution C under stirring state, and continue stirring for 10 hours after the dropwise addition;
[0054] 4) The precipitate obtained in step 3) was centrifuged at 8000 r / min, washed 3 times with deionized water and 2 times with absolute ethanol, and dried in vacuum at 110°C for 24 h to obtain conductive polymer-coated Prussian blue MnHCFe@PEDOT-10, a c...
Embodiment 2
[0060] In this embodiment, the Prussian blue analog MnHCFe is prepared by the same method as that of Comparative Example 1, and the conductive polymer-coated Prussian blue sodium-ion battery cathode material is obtained by in-situ polymerization of the conductive polymer monomer on the surface. The specific steps are:
[0061] 1) Add 200 mg of MnHCFe and 20 μL of EDOT in Comparative Example 1 into 40 mL of deionized water, ultrasonically disperse and stir to obtain cloudy liquid C, and keep at 25 °C;
[0062] 2) Dissolve 40 mg ammonium persulfate in 10 mL deionized water to form solution D;
[0063] 3) Add solution D to turbid solution C under stirring state, and continue stirring for 10 hours after the dropwise addition;
[0064] 4) The precipitate obtained in step 3) was centrifuged at 8000 r / min, washed 3 times with deionized water and 2 times with absolute ethanol, and dried in vacuum at 110°C for 24 h to obtain conductive polymer-coated Prussian blue MnHCFe@PEDOT-20, a c...
Embodiment 4
[0070] In this embodiment, the Prussian blue analog MnHCFe is prepared by the same method as that of Comparative Example 1, and the conductive polymer-coated Prussian blue sodium-ion battery cathode material is obtained by in-situ polymerization of the conductive polymer monomer on the surface. The specific steps are:
[0071] 1) Add 200 mg of MnHCFe and 50 μL of EDOT in Comparative Example 1 into 40 mL of deionized water, ultrasonically disperse and stir to obtain cloudy liquid C, and keep at 25 °C;
[0072] 2) Dissolve 100 mg ammonium persulfate in 10 mL deionized water to form solution D;
[0073] 3) Add solution D to turbid solution C under stirring state, and continue stirring for 10 hours after the dropwise addition;
[0074] 4) The precipitate obtained in step 3) was centrifuged at 8000 r / min, washed 3 times with deionized water and 2 times with absolute ethanol, and dried in vacuum at 110°C for 24 h to obtain conductive polymer-coated Prussian blue MnHCFe@PEDOT-50 is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com