Electrochemical induced mineral deposition system and method
A deposition system and electrochemical technology, applied in the field of electrochemical deposition, can solve problems such as complex and uncontrollable influencing factors, and achieve the effects of controllability, flexible types, and designability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
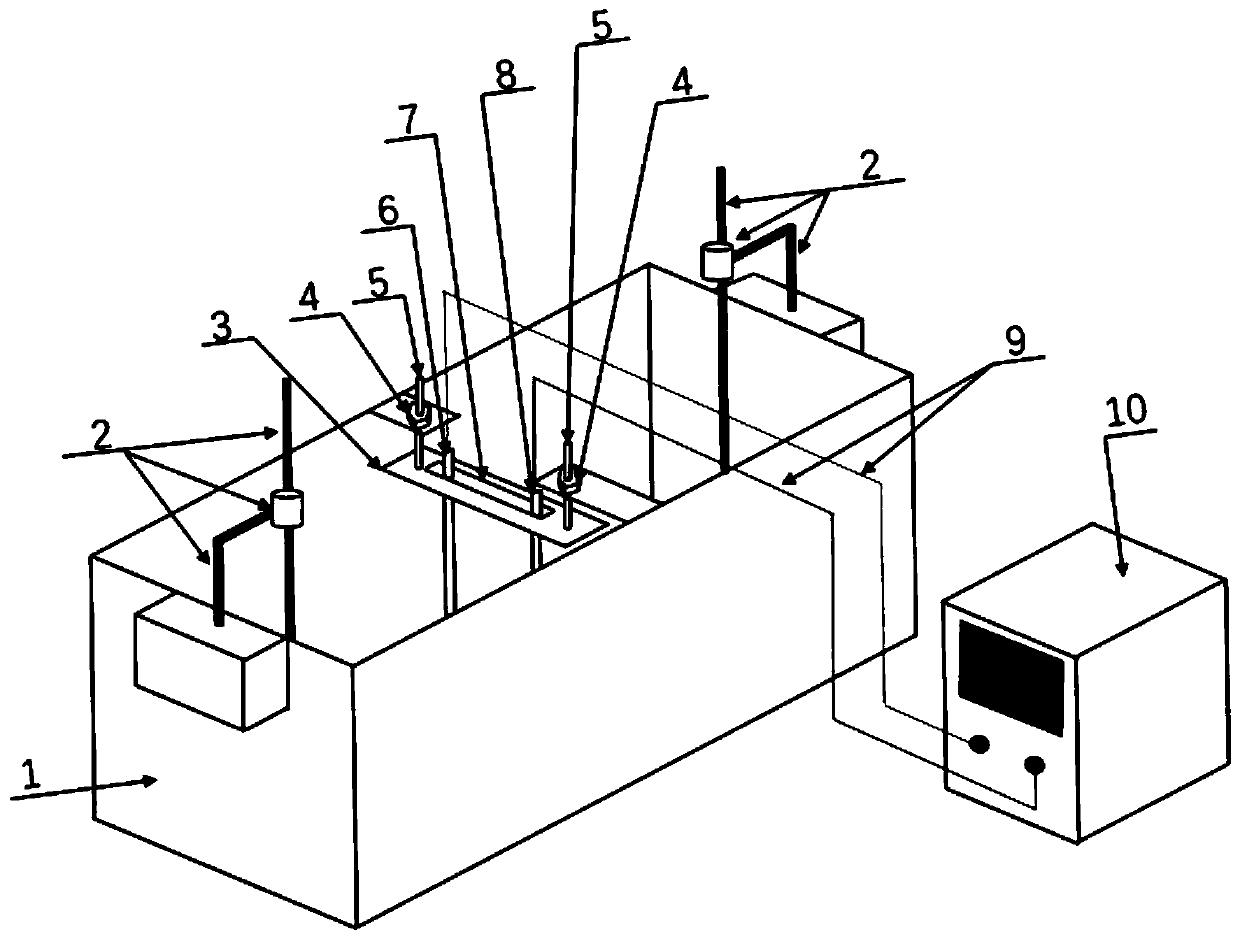
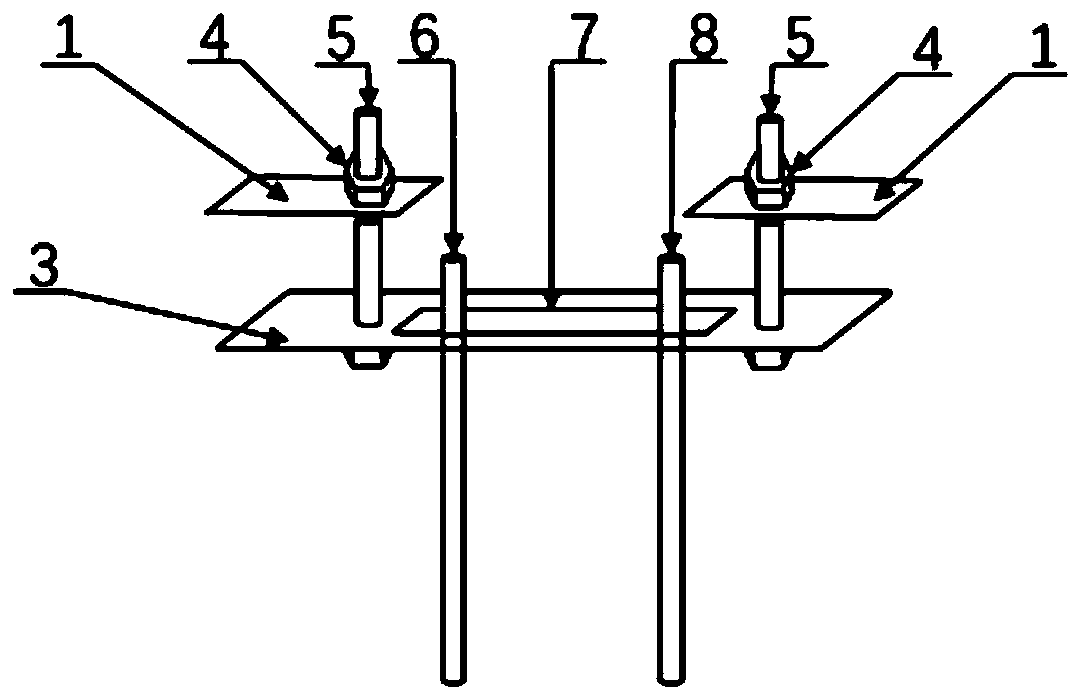
[0047] Select MgCl with a concentration of 0.0125mol / L 2 The solution is an electrolyte solution; the anode material is a ruthenium iridium titanium rod with a diameter of 3mm, the anode material is a 304 stainless steel rod with a diameter of 5mm, the distance between the control cathode and the anode is 40mm, and the depth of the control electrode immersion in the liquid surface is 60mm; external power supply selection Stabilized DC power supply, using constant current mode, the current size is set to 5mA; test the magnesium ion concentration and pH value in the solution every 12h, by adding MgCl 2 and NaOH control the electrolyte concentration and pH to remain basically unchanged; the deposition time is set to 5d. The mass of the deposited product was 0.5649g, the thickness of the deposit was 1.12mm, and the growth rate of the deposit on the cathode was 103.86g·d -1 m -2 .
Embodiment 2
[0049] The seawater solution in the south coast of Qingdao City, Shandong Province is selected as the electrolyte solution; the anode material is a ruthenium iridium titanium rod with a diameter of 3 mm, and the anode material is a 304 stainless steel rod with a diameter of 5 mm. The distance between the control cathode and the anode is 40 mm, and the electrode is immersed in the liquid The surface depth is 60mm; the external power supply is a regulated DC power supply, and the constant current mode is adopted, and the current is set to 5mA; the seawater is replaced every 12h, and the electrolyte concentration and pH are basically kept unchanged; the deposition time is set to 5d. The mass of the deposited product was 0.9185g, the thickness of the deposit was 1.98mm, and the growth rate of the deposit on the cathode was 134.89g·d -1 m -2 .
Embodiment 3
[0051] Select MgCl 2 The concentration is 0.05mol / L and CaCl 2 A mixed solution with a concentration of 0.01mol / L is used as the electrolyte solution; the anode material is a ruthenium iridium titanium rod with a diameter of 3 mm, and the anode material is a 304 stainless steel rod with a diameter of 5 mm. The distance between the cathode and the anode is controlled to be 40 mm, and the electrode is immersed in the liquid The surface depth is 60mm; the external power supply selects a regulated DC power supply, adopts the constant current mode, and the current size is set to 20mA; the magnesium ion concentration and pH value in the solution are tested every 12h, and the concentration of magnesium ions in the solution is tested by adding MgCl 2 , CaCl 2 and NaOH control the electrolyte concentration and pH to remain basically unchanged; the deposition time is set to 5d. The mass of the deposited product was 2.2941g, the thickness of the deposit was 3.16mm, and the growth rate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com