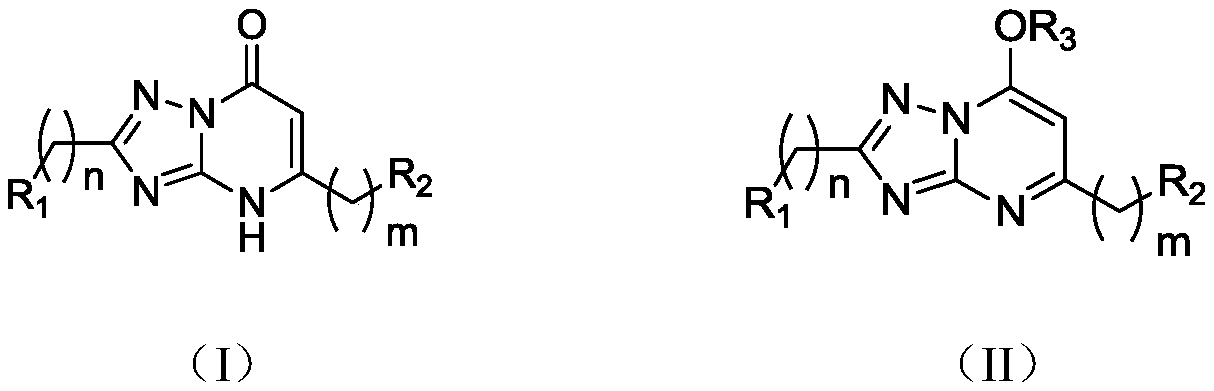
Application of 1,2,4-triazole heterocyclic compound in preparation of drugs to prevent or treat central system related diseases
A technology of heterocyclic compounds and triazoles, which is applied in the field of 1,2,4-triazole heterocyclic compounds, can solve the problems of not showing epileptic central nervous excitatory effects, and achieve good clinical application prospects and good sedation , Excellent inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Add 2.0mmol of aminoguanidine carbonate, 30mL of dichloromethane, and 4.0mmol of pyridine into the reaction flask, cool the resulting mixture to 0°C, add dropwise 10mL of dichloromethane solution in which 1.0-2.0mmol of acid chloride is dissolved, and the dropwise addition is complete Afterwards, the resulting reaction mixture was slowly raised to room temperature and reacted at room temperature for 1-12 hours. After the reaction was completed, the reaction solution was concentrated to dryness and then directly proceeded to the next step of reaction, or adding water to dilute the residue and using dilute sodium hydroxide solution Slowly adjust the pH value of the solution to about 12, and a large amount of solids are precipitated during the addition of alkali, and filtered; the filter cake is washed and dried with a small amount of ice water, suspended or dissolved in water, and a catalytic amount of p-toluenesulfonic acid is added, and the resulting mixture is heated to ...
Embodiment 2
[0039] Add 2.0mmol aminoguanidine carbonate and 1.0mmol or 2.0mmol carboxylic acid (carboxylate) in the reaction flask, add 30mLN, N-dimethylformamide and 1.0 equivalent of pyridine, the resulting reaction mixture is heated to 100- React at 120°C for 12 hours. After the reaction, concentrate under reduced pressure and evaporate the solvent to dryness. Add water to the obtained residue. Part of the compound precipitates as a solid. After filtering, the obtained filter cake is washed with a small amount of ice water and dried. Derivatives of ethyl acetate are dissolved in butanol, a catalytic amount of p-toluenesulfonic acid is added, the resulting mixture is heated to reflux and reacted at this temperature for 12-48 hours, after the reaction is completed, the solvent is removed by concentrating under reduced pressure, and the obtained residue The product was purified by column chromatography to obtain [1,2,4]-triazolo[1,5-a]pyrimidinone heterocyclic compound. Table 1 lists the ...
Embodiment 3
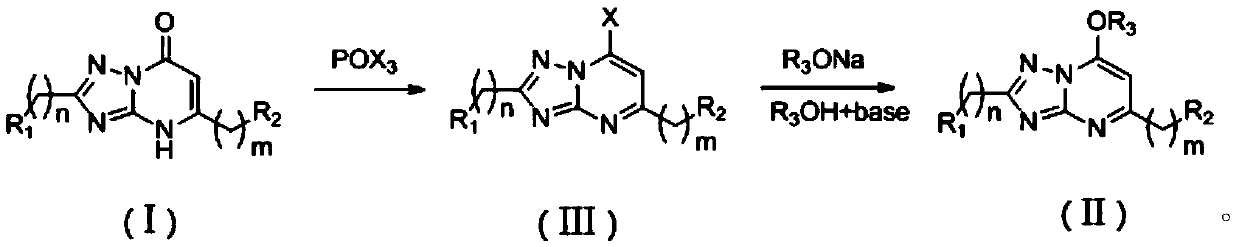
[0045] Add the [1,2,4]-triazolo[1,5-a]pyrimidinone compound 1-31.6mmol in Example 1 to 10ml of phosphorus oxychloride solution, and then add 4 drops of N,N- Dimethylformamide, reflux at 100°C for 5 hours and then cool to room temperature, add ice water and ethyl acetate to the reaction solution and stir for 10 minutes, separate the ethyl phase, and extract the water phase with ethyl acetate 2- 3 times, collect the ethyl acetate layer and evaporate to dryness to obtain 7-chloro-[1,2,4]-triazolo[1,5-a]pyrimidine derivative; add 1.24mmol sodium hydride to 10ml alcohol at 0°C , after complete dissolution, add 0.31mmol 7-chloro-[1,2,4]-triazolo[1,5-a]pyrimidine derivatives, react for 0.5 hours, then transfer to room temperature for 0.5-2 hours, then react Add water and dichloromethane to the liquid, separate the dichloromethane phase, extract the water phase with dichloromethane 2-3 times, collect the dichloromethane layer, and evaporate to dryness to obtain 7-alkoxy-1,2,4- Triazo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com