Fluorescent liquid crystal monomer and polymer containing α-cyano-stilbene structure and preparation method thereof
A technology containing cyano stilbene and liquid crystal monomers, applied in liquid crystal materials, chemical instruments and methods, luminescent materials, etc., can solve the problems of increasing the complexity and difficulty of devices, poor film-forming properties of small molecular materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
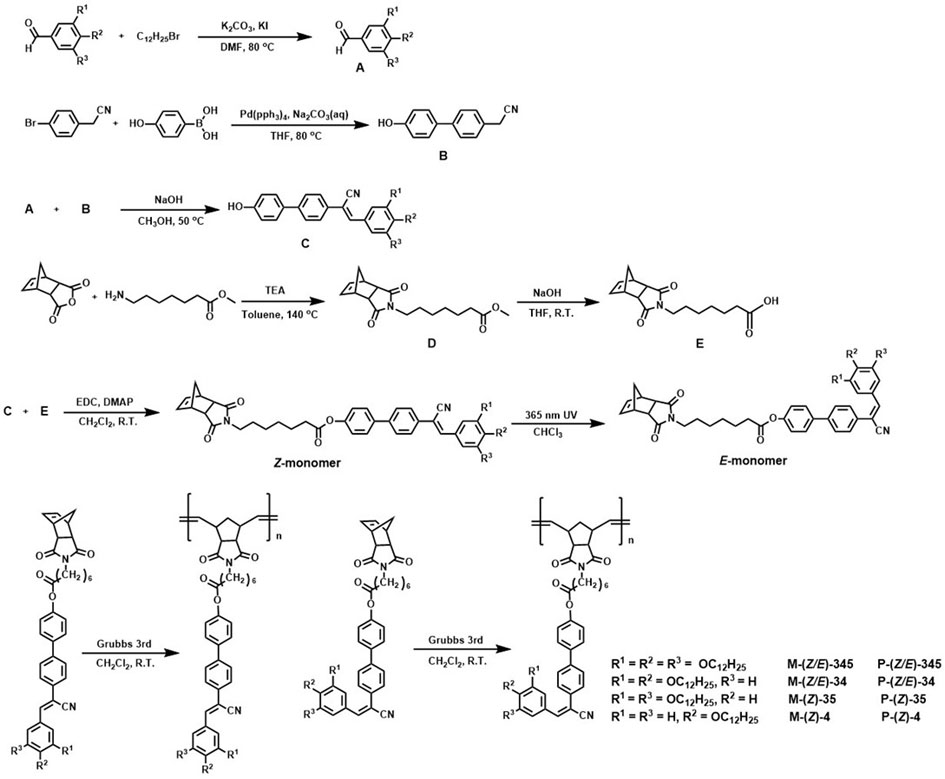
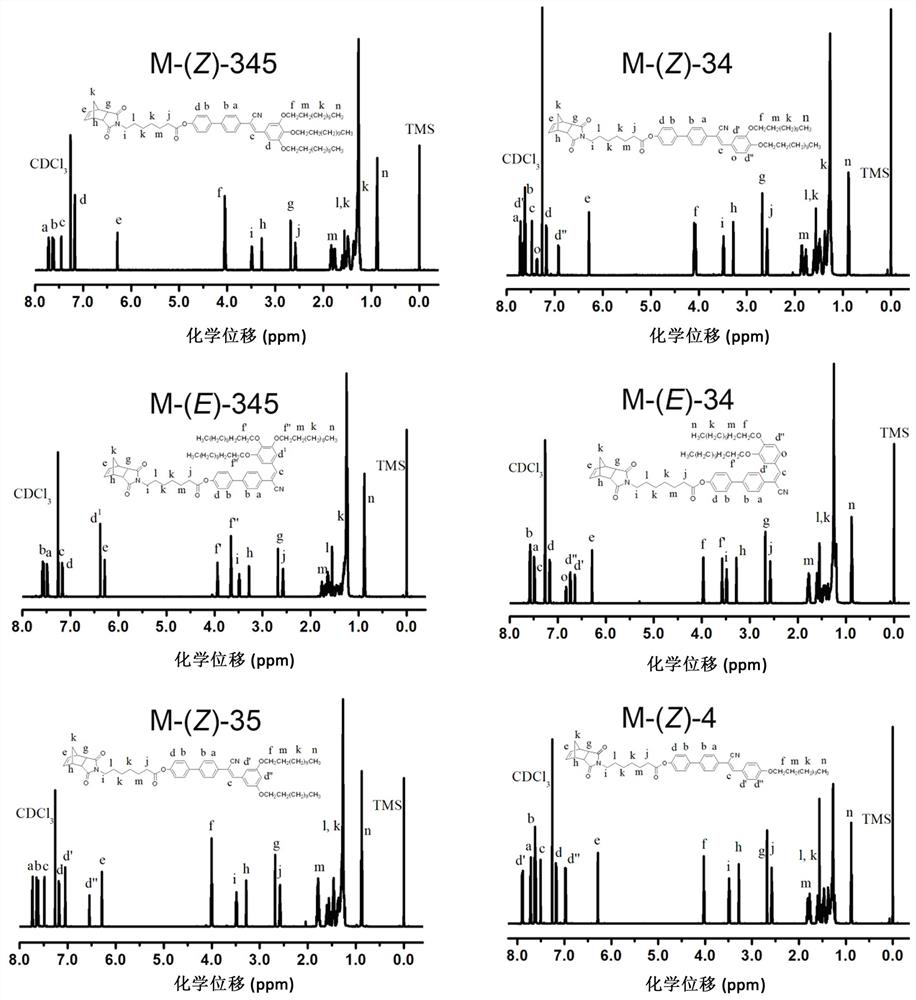
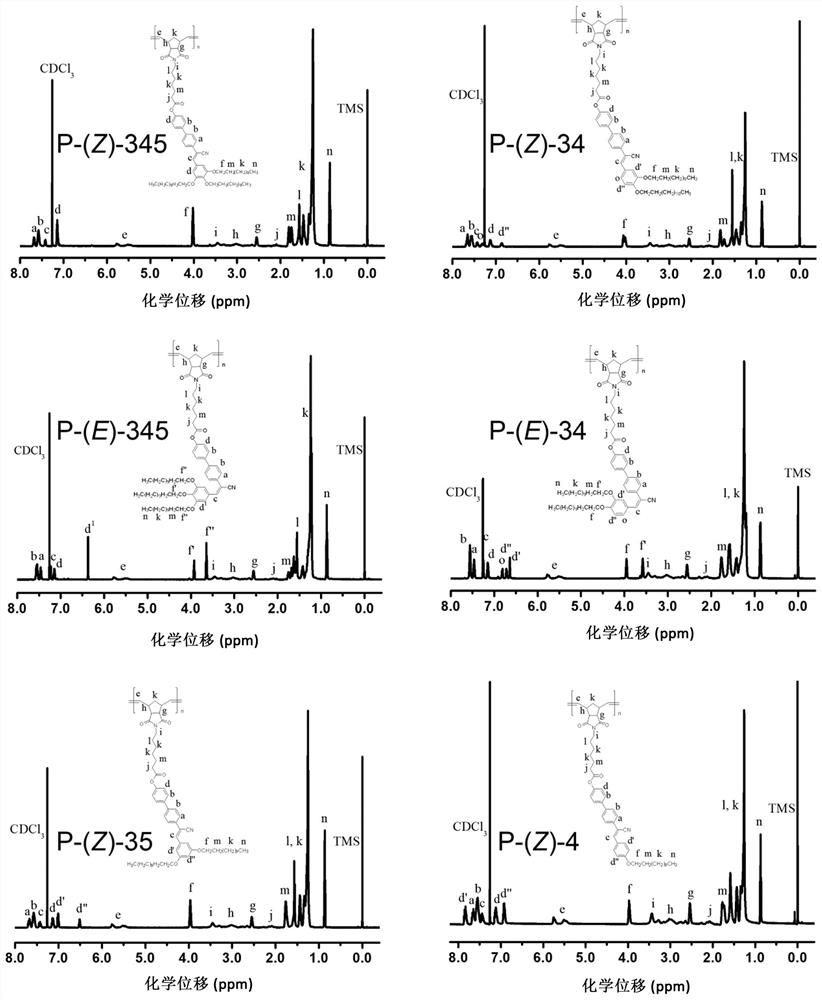
[0061] See attached figure 1 , it is the synthesis route diagram of the fluorescent liquid crystal monomer and polymer of alpha-cyano-stilbene structure of the present invention; In this embodiment, the fluorescent liquid crystal monomer and polymer of alpha-cyano-stilbene structure Prepared to R 1 =R 2 =R 3 = OC 12 h 25 The corresponding monomers and polymers are taken as examples, mainly including the following steps:
[0062] Step 1, preparation of compound A: 2.00 (11.62 mmol) of 3,4,5-trihydroxybenzaldehyde was dissolved in 40 mL of dry N, N-dimethylformamide, 5.62 g (40.66 mmol) of potassium carbonate was added and Potassium iodide (amount of catalyst), at 80 o 11.24 mL of bromododecane (46.48 mmol) was added dropwise under stirring, and the reaction was continued for 24 h after the addition was complete. After the reaction, when the system was down to room temperature, the mixture was poured into brine and stirred continuously, then extracted with dichlorometha...
Embodiment 2
[0076] For the monomers and polymers prepared in Example 1, take 1 mg samples and place them between two cell slides for polarizing microscope testing. Raise the samples to the isotropic temperature and take photos during the cooling process. For the results, see the attached Figure 4 ,all Z -isomer, whether it is a polymer or a monomer, they all have a liquid crystal texture, indicating that they have liquid crystallinity, and for E -isomer, no liquid crystal texture appeared during the whole cooling process, indicating no liquid crystallinity.
Embodiment 3
[0078] With the polymer prepared in Example 1, take 5 mg samples and wrap them in aluminum foil for small angle X-ray scattering test, the results are shown in the attached Figure 5 . P-( Z )-345 placed at 170 o The samples were piled up on the hot stage C, and then the hot stage was turned off to allow it to gradually cool down to room temperature, and the values of the scattering vectors that appeared were 1.00, 1.76, and 2.00 nm in turn -1 , and their ratio is 1: : 2, is a hexagonal columnar phase structure. P-( Z )-34 placed at 200 o The samples were piled up on the hot stage C, and then the hot stage was turned off to allow it to gradually cool down to room temperature, and the values of the scattering vectors that appeared were 1.01, 2.03 nm in turn -1 , and their ratio is 1:2, which is a smectic phase structure. P-( Z )-35 placed at 120 o The samples were piled up on the hot stage C, and then the hot stage was turned off to allow it to gradually cool down...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com