Norovirus gⅰ, gⅱ and gⅳ type nucleic acid typing detection kit and detection method
A technology for detecting kits and viral nucleic acids, which is applied to biochemical equipment and methods, and microbial measurement/inspection. , the effect of reducing workload
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1——Norovirus GⅠ, GⅡ, GⅣ nucleic acid typing detection kit
[0046] A kit for multiple detection of Norovirus GⅠ, GII and GIV types by real-time fluorescent RT-PCR, including: virus nucleic acid rapid extraction reagents, PCR amplification reagents, PCR enzyme mixture, Norovirus GⅠ, GⅡ and GIV types Nucleic acid detection reagents, positive control substances, negative control substances. in:
[0047] (1) The viral nucleic acid rapid extraction reagent includes a lysis reagent, and the lysis reagent comprises: 100mM Tris-HCl with a pH of 10.0, 5% v / v Tween20, 4M guanidine isothiocyanate, 50mM KCl, and 2mM EDTA with a pH of 8.0.
[0048] (2) The PCR amplification reagent includes PCR amplification buffer; specifically includes: 2×PCR buffer, 50mM MgCl 2 , 100 mM Tris-HCl, pH 10.0, 0.5 mM dNTPs, 5% g / mL BSA.
[0049] (3) The PCR enzyme mixture includes 0.5 U / μl reverse transcriptase, 5 U / μl DNA polymerase, and 5×RNase inhibitor.
[0050] (4) The Norovirus GI, G...
Embodiment 2
[0053] Example 2——Norovirus GⅠ, GⅡ and GⅣ type nucleic acid typing detection method
[0054] A real-time fluorescent RT-PCR multiple typing detection method for Norovirus GⅠ, GII and GIV types, comprising the following experimental steps:
[0055] (1) Main reagents and instruments: the kit reagents in Example 1 were used; the fluorescent quantitative PCR instrument was ABI7500.
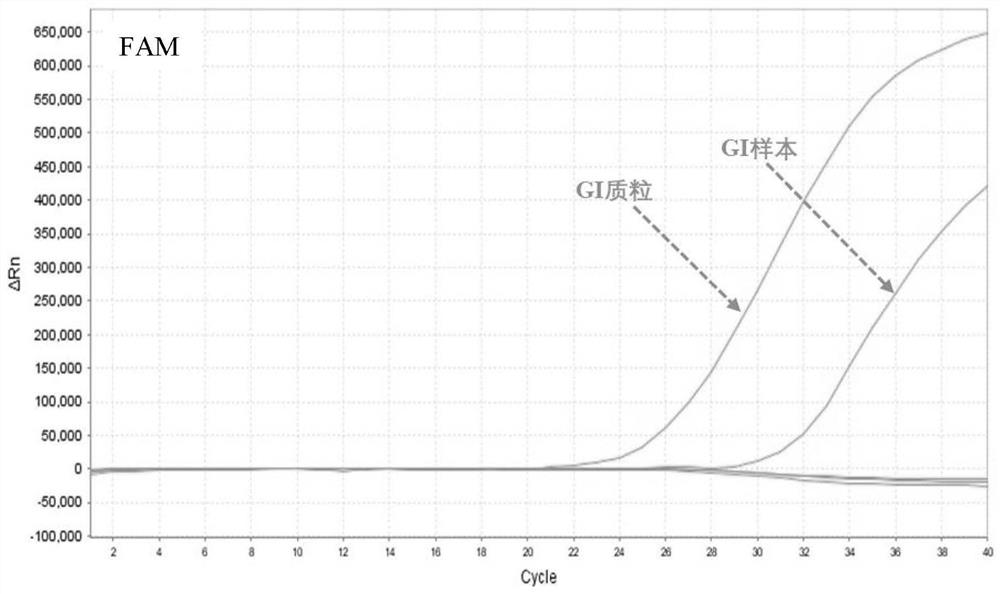
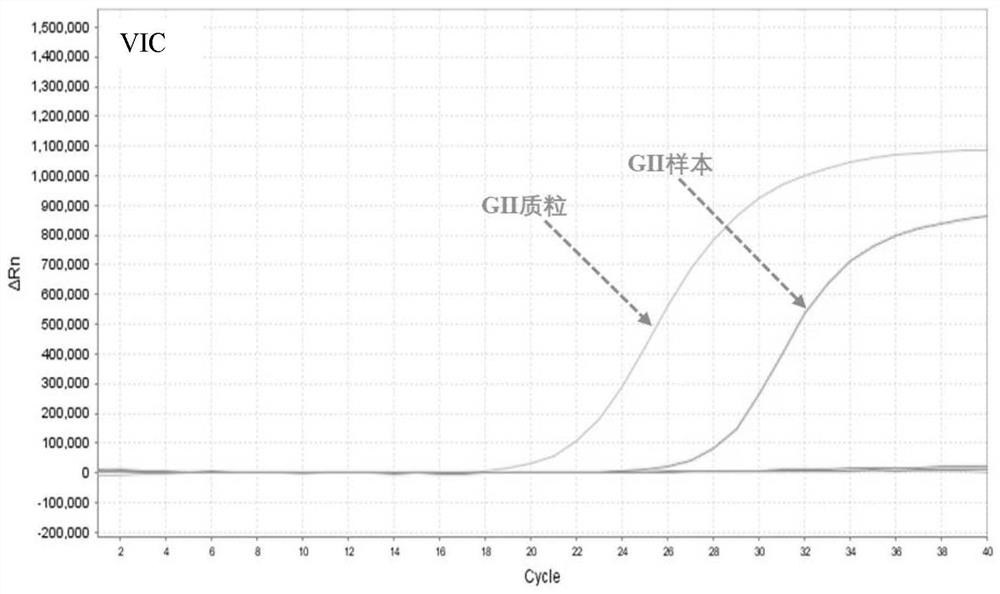
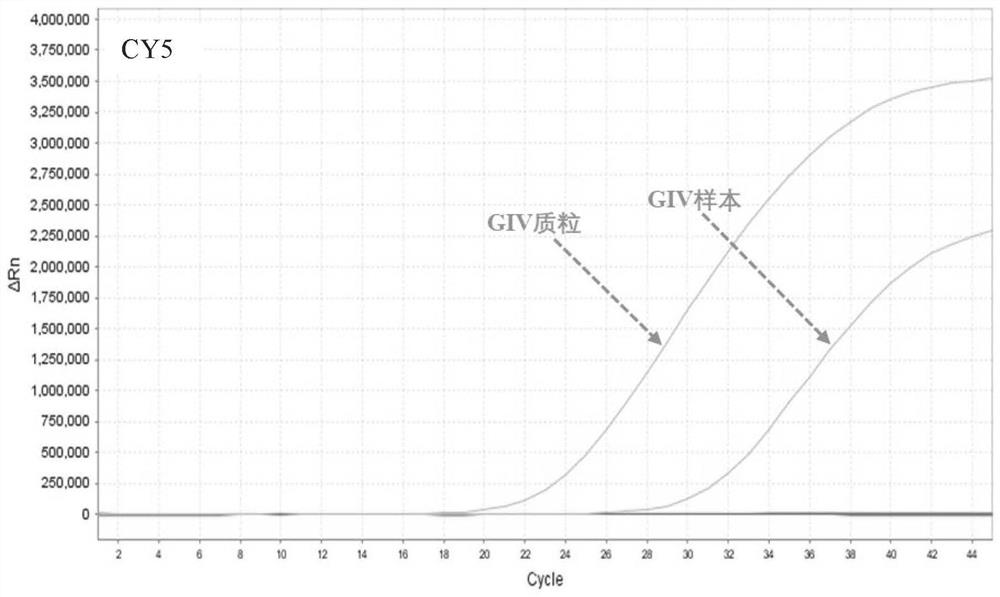
[0056] (2) Specimen preparation: the positive samples were norovirus GⅠ positive patient samples, norovirus GⅡ positive patient samples, norovirus GIV positive patient samples, and then diluted with DEPC water in different multiples, negative control The specimens were samples from patients negative for norovirus GⅠ, GⅡ and GⅣ infection.
[0057] (3) Nucleic acid extraction: Mix equal volumes of the viral nucleic acid rapid extraction reagent and the sample to be tested, let stand at room temperature for 5-10 minutes, and then directly use the lysed mixture for PCR detection.
[0058] (4) Specific R...
Embodiment 3
[0094] Example 3——verify the influence of KCl in the rapid extraction reagent of viral nucleic acid on the detection effect of fluorescent PCR
[0095] (1) According to the formula provided by the present invention, the viral nucleic acid rapid extraction reagent A containing KCl is prepared: pH is 10.0 100mM Tris-HCl, 5%v / v Tween20, 4M guanidine isothiocyanate, 50mM KCl, pH 8.0 2 mM EDTA.
[0096] (2) Prepare KCl-free viral nucleic acid rapid extraction reagent B: 100 mM Tris-HCl with a pH of 10.0, 5% v / v Tween20, 4M guanidine isothiocyanate, and 2 mM EDTA with a pH of 8.0.
[0097] (3) Using norovirus GⅠ, GⅡ and GⅣ positive clinical samples, mix the virus nucleic acid rapid extraction reagent A and viral nucleic acid rapid extraction reagent B with equal volumes of the sample respectively, let stand at room temperature for 5-10 minutes, and then lyse and mix Solutions A and B were used for PCR detection respectively.
[0098] (4) The norovirus GⅠ, GII and GIV fluorescent P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com