Molecular marker for diagnosing idiopathic inflammatory myopathy and application thereof
A molecular marker and idiopathic technology, applied in the field of medicine and biology, can solve problems such as modification, time-consuming, and inability to translate proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
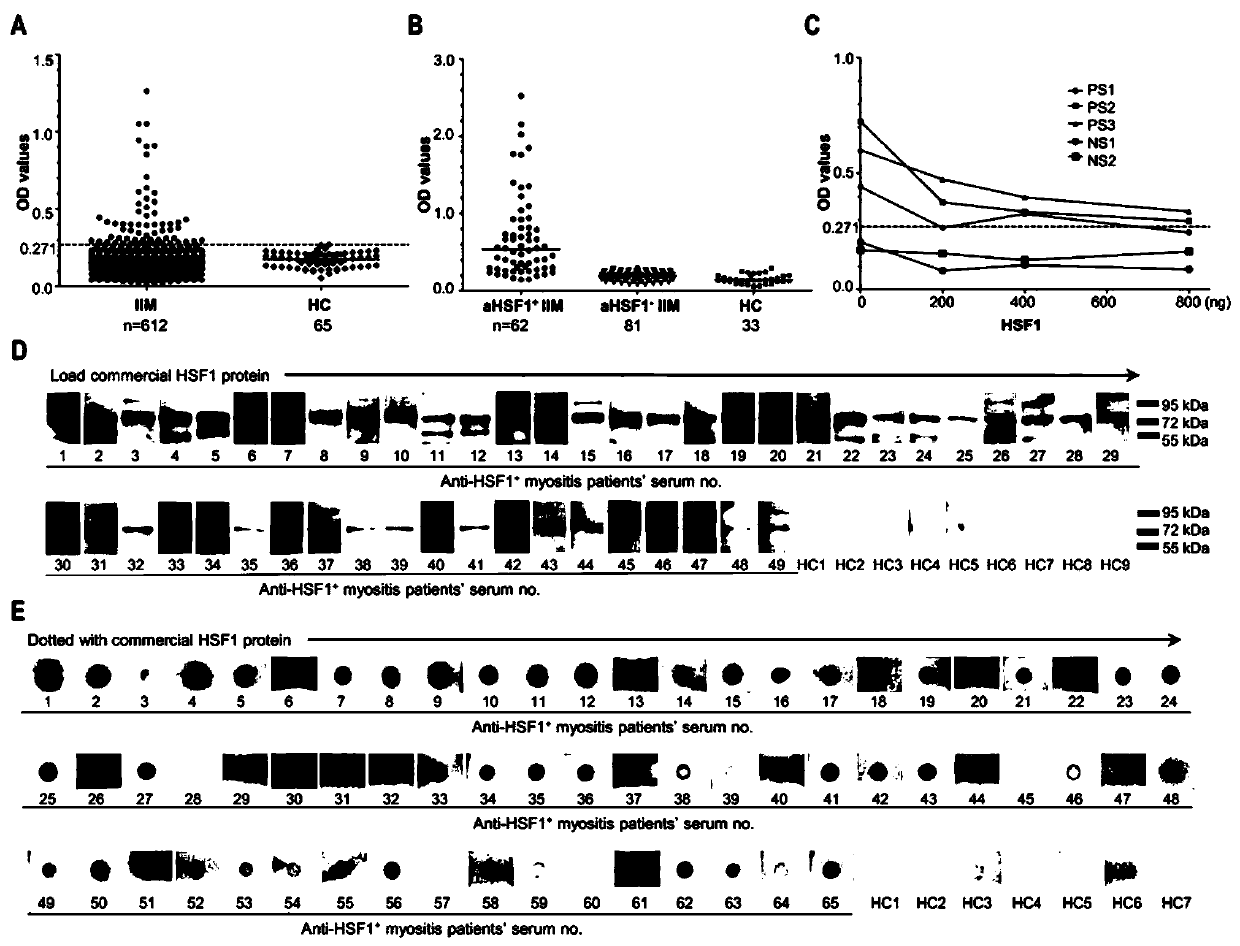
[0091] Example 1. Screening for highly specific antibodies in IIM and determining the presence of HSF1 antibodies
[0092] application Human Protein Microarray v5.1 (Life Technologies, USA) detected the distribution of antibodies in the sera of 10 DM patients and 10 HCs, and performed statistical analysis. The specific method is:
[0093] ① Blocking, configure blocking solution (50mM HEPES, 200mM NaCl, 0.01% Triton X-100, 25% glycerol, 20mM reduced glutathione, 1.0mM DTT, 1X Synthetic Block), put the chip in the chip incubation box and block for 1h at 4°C ;
[0094] ② For serum sample incubation, add the prepared sample diluent (1:500; 10 cases of DM patients, 10 cases of HCs) into the chip incubation box, and incubate at 4°C for 1.5h;
[0095] ③Secondary antibody incubation, Alexa Add 647-conjugated goat anti-human IgG fluorescent secondary antibody to the chip incubation box, and incubate at 4°C in the dark for 1.5h;
[0096] ④ Drying, use a chip centrifuge to centrif...
Embodiment 2
[0100] Embodiment 2, ELISA detection anti-HSF1 antibody
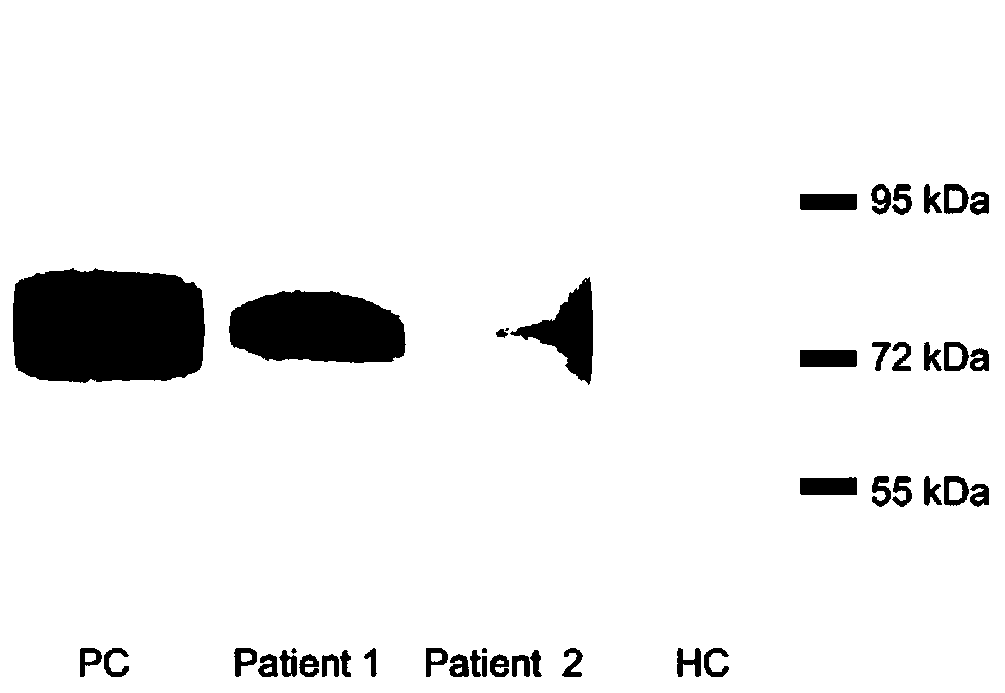
[0101] The commercially available recombinant HSF1 protein (Abcam, Cambridge, UK) was diluted and coated in the reaction wells (200ng / well) of a polystyrene plate (Thermo Scientific, Roskilde, Denmark), and the level of anti-HSF1 antibody in serum was detected by ELISA. At the same time, another commercially available recombinant HSF1 protein (OriGene, Maryland, USA) ELISA was used to detect the level of anti-HSF1 antibody in serum. Serial dilution ELISA (1:2) was used to determine the optimal dilution ratio of serum, and ELISA blocking experiment was used to determine the specificity of ELISA for detecting anti-HSF1 antibody. All serum samples were tested twice. Before detecting serum anti-HSF1 antibody by ELISA, the diluted serum of anti-HSF1 positive IIM patients (3 cases) and HCs (2 cases) was incubated with recombinant HSF1 protein (Abcam, Cambridge, UK) overnight at 4°C, and then the anti-HSF1 antibody was detect...
Embodiment 3
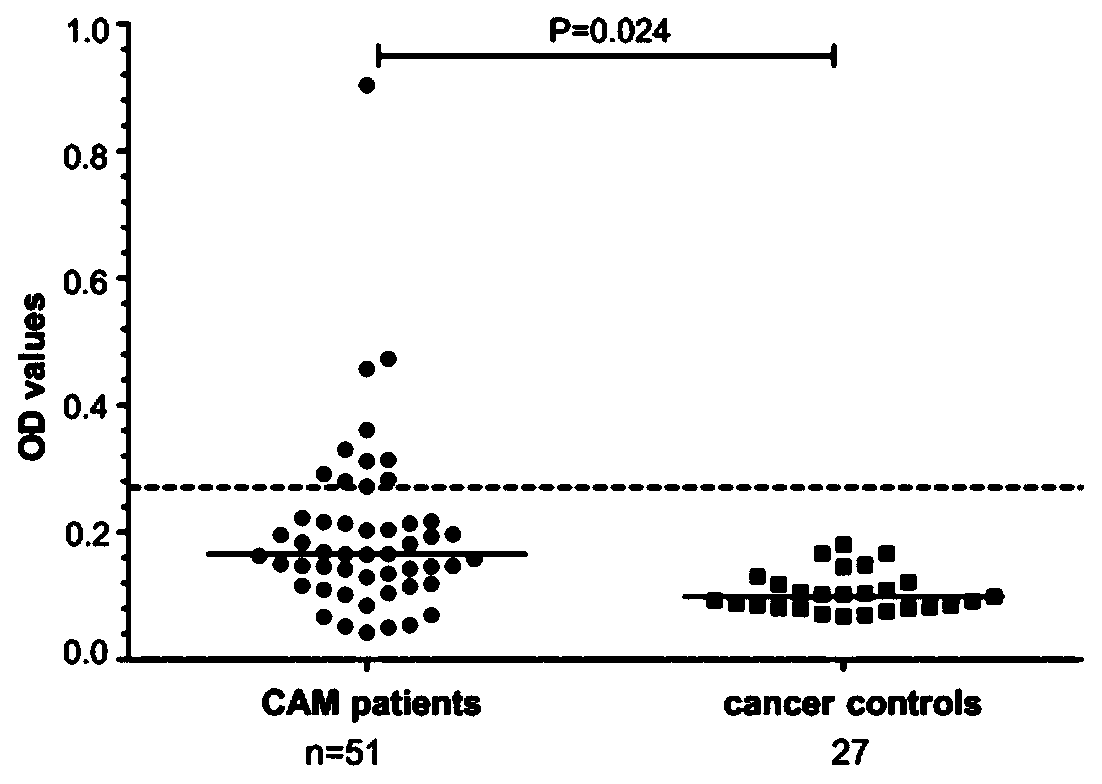
[0104] Example 3, clinical features of anti-HSF1 positive IIM patients
[0105] Anti-HSF1 positive IIM patients had a higher proportion of CAM (16.9% vs 7.3%, P=0.008), and common weight loss (P=0.046) and pruritus (P=0.039). Meanwhile, anti-HSF1 antibody was correlated with anti-TIF1γ antibody (P=0.046), hyperglobulinemia (P<0.001), elevated C-reactive protein (P=0.039) and elevated erythrocyte sedimentation rate (ESR, P=0.006). We did not find that anti-HSF1 antibodies were associated with other typical IIM clinical features, such as skin involvement, pulmonary interstitial lesions, and elevated muscle enzymes (Table 2). In addition, the karyotype of ANA in anti-HSF1-positive IIM patients' serum was mainly speckled or cytoplasmic granular.
[0106] Table 2 Clinical characteristics of anti-HSF1 antibody positive IIM patients
[0107]
[0108]
[0109] HSF1, heat shock transcription factor 1; IIM, idiopathic inflammatory myopathy; PM, polymyositis; DM, dermatomyositis; A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com