Metallic oxide catalyst with high stability performance and preparation thereof
A technology of oxides and catalysts, which is applied in the field of metal oxide catalysts and their preparation, can solve the problems of substitutes for precious metal catalysts, increase the specific surface area of catalysts, and do not have high specific surface areas, so as to achieve low prices and improve high-temperature hydrothermal Effects of stability and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
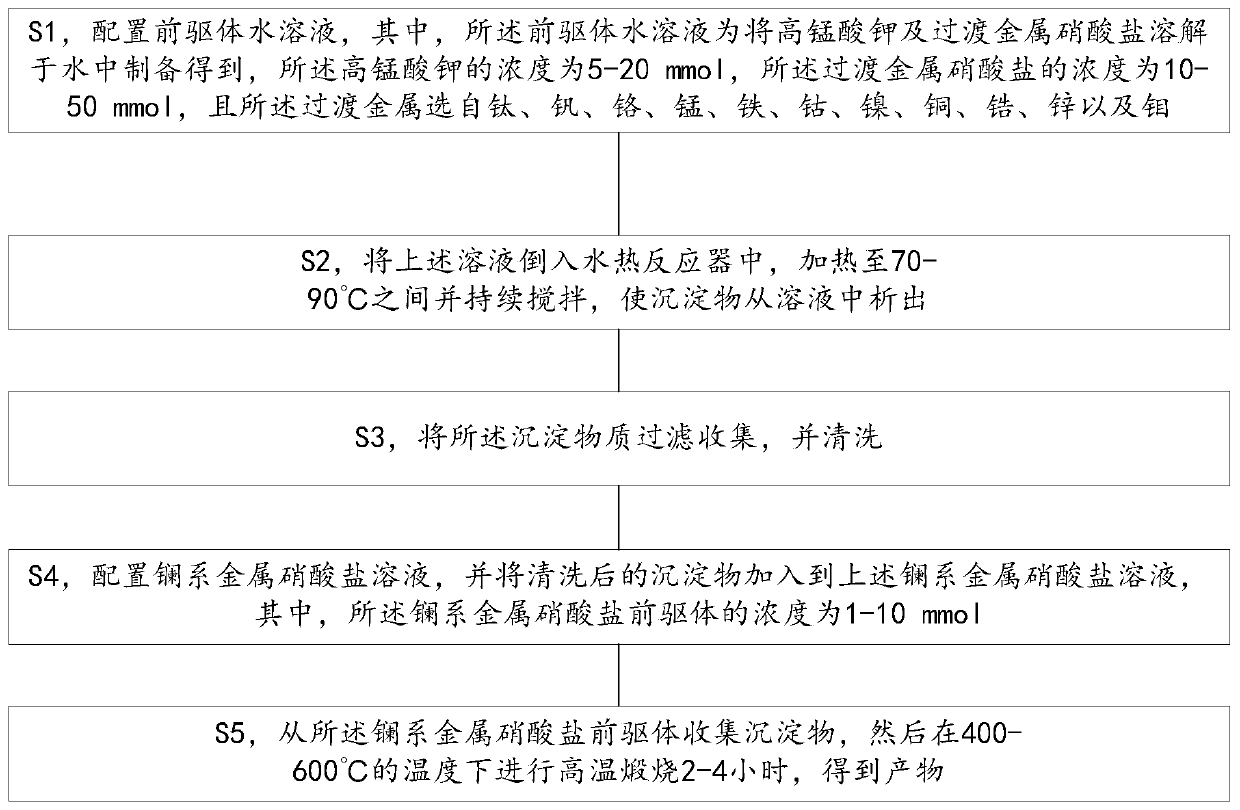
[0017] refer to figure 1 Shown, a kind of preparation method with the metal oxide catalyst of high stability comprises the following steps:
[0018] S1, configuring an aqueous precursor solution, wherein the aqueous precursor solution is prepared by dissolving potassium permanganate and transition metal nitrate in water, the concentration of the potassium permanganate is 5-20 mmol, and the transition metal nitrate The concentration is 10-50mmol, and the transition metal is selected from titanium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, zirconium, zinc and molybdenum;
[0019] S2, pour the above solution into a hydrothermal reactor, heat to 70-90°C and keep stirring to precipitate the precipitate from the solution;
[0020] S3, collecting the precipitated substance by filtration, and cleaning;
[0021] S4, configuring a lanthanide metal nitrate solution, and adding the washed precipitate to the above lanthanide metal nitrate solution, wherein the concentr...
Embodiment 1
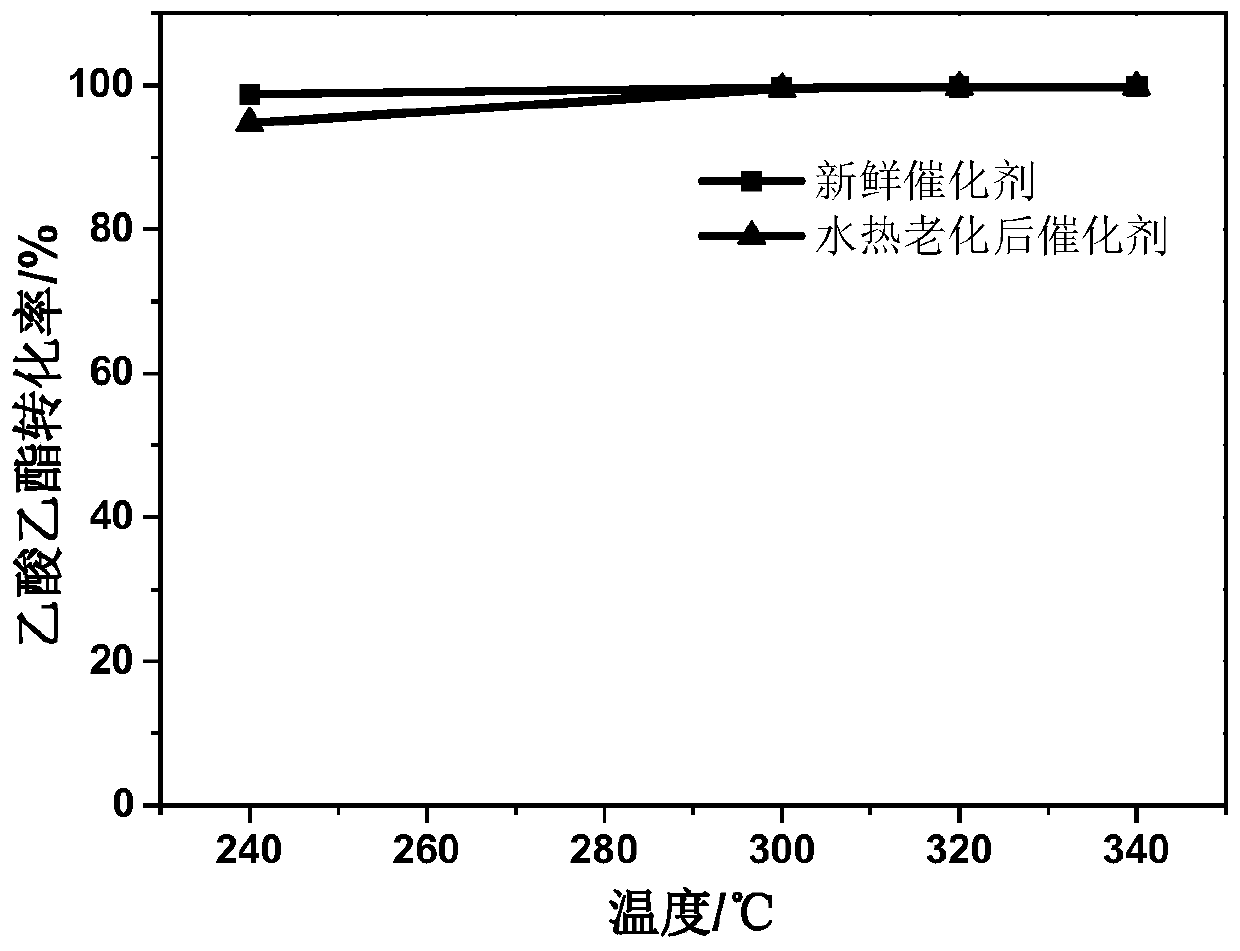
[0029] Configure 1 liter of mixed solution of potassium manganate and cobalt nitrate as the precursor solution of hydrothermal reaction, wherein the concentration of potassium permanganate is 15mmol, and the concentration of cobalt nitrate is 40mmol; the solution is poured into the hydrothermal reactor, and the solution is heated to 85°C and keep stirring, as the reaction proceeds, the obtained manganese cobalt oxide will precipitate out of the solution in the form of precipitation; the precipitate produced in the solution is collected by filtration and cleaned with deionized water; the cleaning The final precipitated matter is dried, and the black product obtained is manganese-cobalt oxide; configure lanthanum nitrate soaking aqueous solution 50ml, the solution concentration is 1-10mmol, immerse manganese-cobalt oxide in the lanthanum nitrate aqueous solution, and let it stand for 40 minutes; collect The manganese-cobalt oxide in the lanthanum nitrate solution is calcined at 4...
Embodiment 2
[0031] It is basically the same as Example 1, except that the transition metal is titanium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com