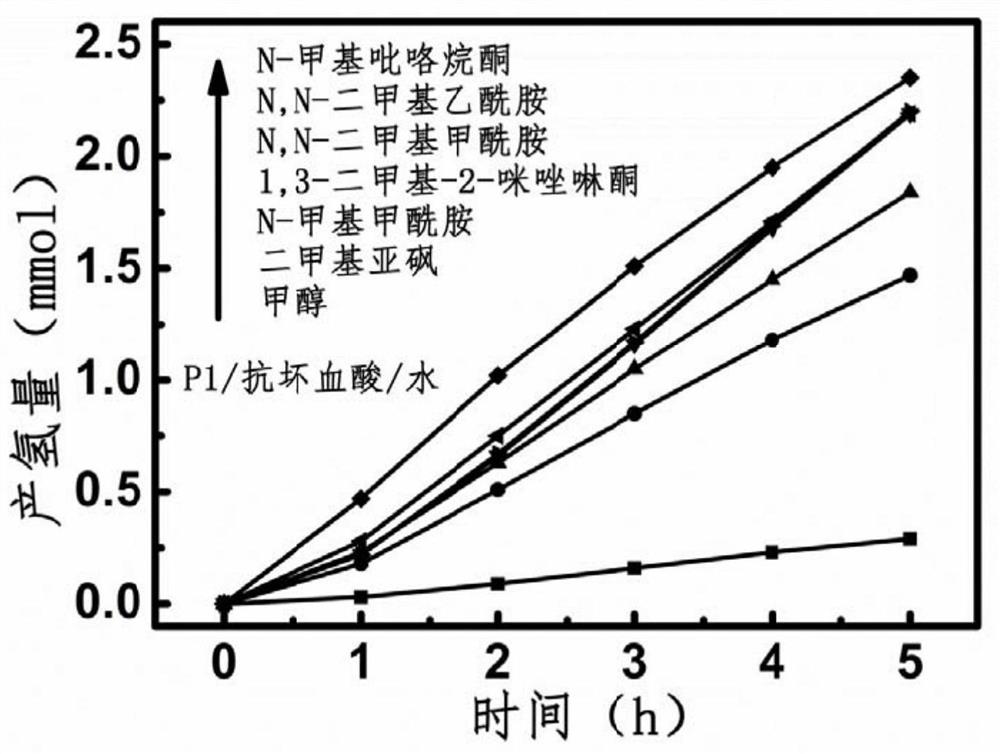
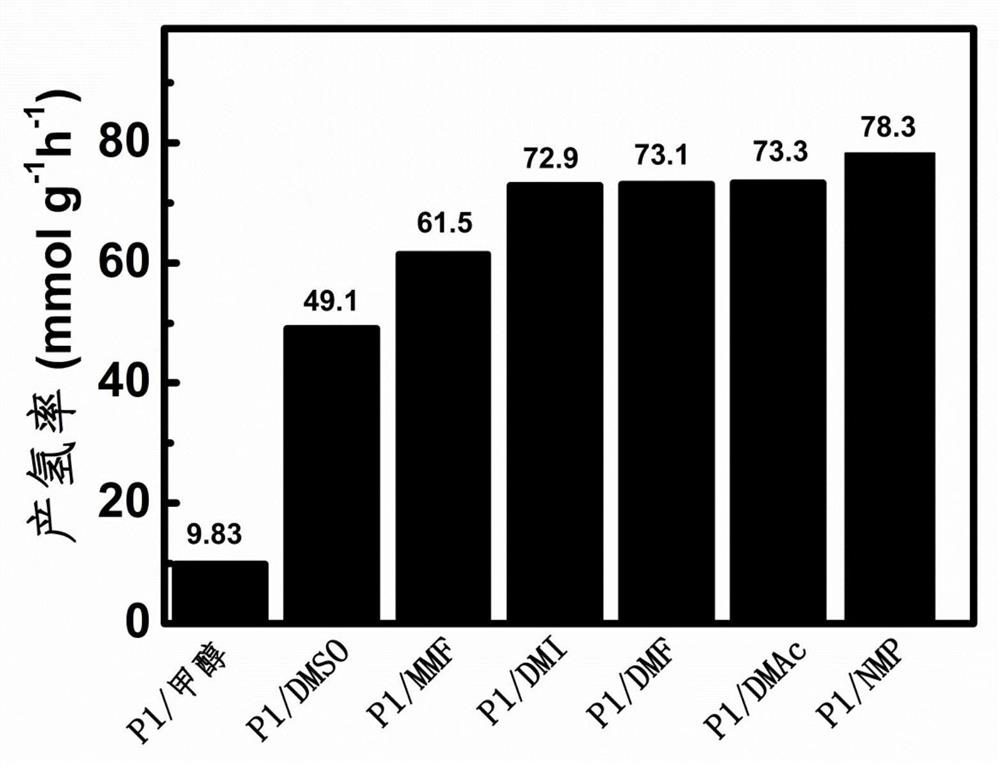
A method based on conjugated porous organic photocatalysts to efficiently split water to produce hydrogen
A photocatalyst, water splitting technology, applied in organic compound/hydride/coordination complex catalysts, chemical instruments and methods, physical/chemical process catalysts, etc. Accuracy, hydrogen source cannot be determined whether it comes from methanol, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
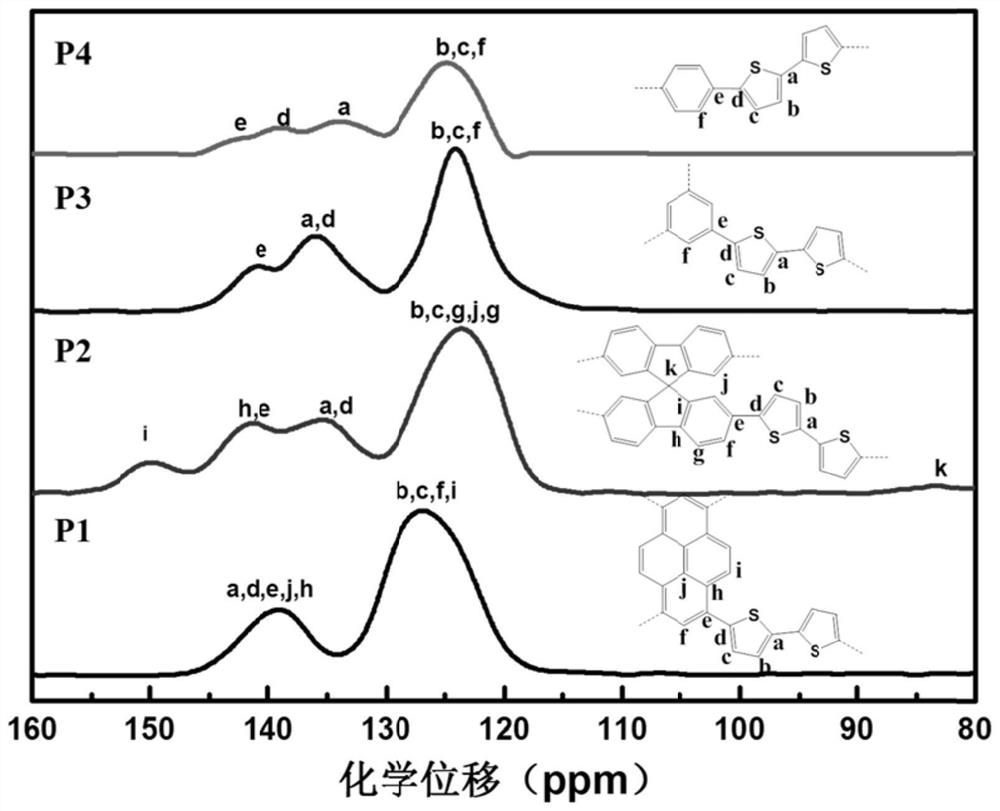
[0037] Preparation Example 1 Preparation of Conjugated Porous Organic Photocatalyst P1
[0038] In a 25mL Schlenk tube, add 1,3,6,8-tetrabromopyrene (0.386mmol, 200.0mg), 5,5′-bis(trimethyltinyl)-2,2′-bithiophene (0.772mmol , 379.9mg), P(o-MeOPh) 3 (0.039mmol, 10.9mg), Pd 2 dba 3 (0.015mmol, 14.1mg) and 10ml of a mixture of toluene / DMF (19:1, v / v). The mixture was degassed under the thawing of the refrigeration pump, purged with argon, stirred at 110°C for 48h, cooled to room temperature and poured into CH 2 Cl 2 , and filtered to obtain the crude product. The resulting crude product was extracted and separated, and CH was added successively. 2 Cl 2 , methanol, NaF solution, water and methanol to purify, remove inorganic salts, unreacted raw materials and other impurities in the reactant, and vacuum-dry the resulting solid product to obtain a conjugated porous organic semiconductor material, denoted as P1.
preparation example 2
[0039] Preparation Example 2 Preparation of Conjugated Porous Organic Photocatalyst P2
[0040] In a 25 mL Schlenk tube, add 2,2′,7,7′-tetrabromospirofluorene (0.386 mmol, 243.9 mg), 5,5′-bis(trimethyltinyl)-2,2′-bithiophene (0.772 mmol, 379.9 mg), P(o-MeOPh)3 (0.039 mmol, 10.9 mg), Pd2dba3 (0.015 mmol, 14.1 mg) and 10 ml of a mixture of toluene / DMF (19:1, v / v). The mixture was degassed under the thawing of the refrigeration pump, purged with argon, stirred at 110°C for 48h, cooled to room temperature and poured into CH 2 Cl 2 , and filtered to obtain the crude product. The resulting crude product was extracted and separated, and CH was added successively. 2 Cl 2 , methanol, NaF solution, water and methanol to purify, remove inorganic salts, unreacted raw materials and other impurities in the reactant, and vacuum-dry the resulting solid product to obtain a conjugated porous organic semiconductor material, denoted as P2.
preparation example 3
[0041] Preparation Example 3 Preparation of Conjugated Porous Organic Photocatalyst P3
[0042] In a 25mL Schlenk tube, add 1,3,5-tribromobenzene (0.515mmol, 162.0mg), 5,5′-bis(trimethyltinyl)-2,2′-bithiophene (0.772mmol, 379.9 mg), a mixture of P(o-MeOPh)3 (0.039mmol, 10.9mg), Pd2dba3 (0.015mmol, 14.1mg) and 10ml of toluene / DMF (19:1, v / v). The mixture was degassed under the thawing of the refrigeration pump, purged with argon, stirred at 110°C for 48h, cooled to room temperature and poured into CH 2 Cl 2 , and filtered to obtain the crude product. The resulting crude product was extracted and separated, and CH was added successively. 2 Cl 2 , methanol, NaF solution, water and methanol purification to remove inorganic salts, unreacted raw materials and other impurities in the reactant, vacuum-dry the resulting solid product to obtain a conjugated porous organic semiconductor material, denoted as P3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com