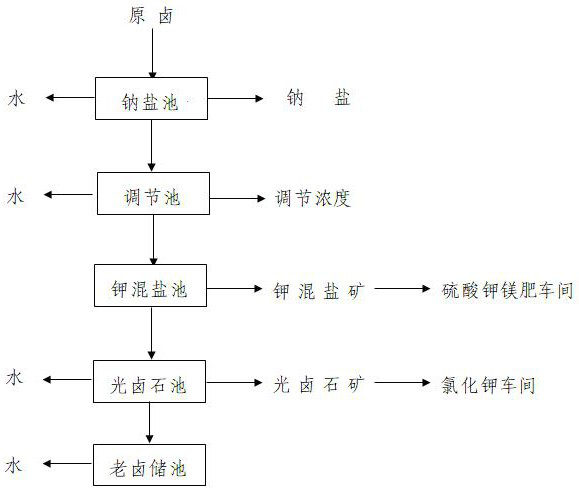
A kind of magnesium sulfate subtype salt lake brine salt field natural evaporation staged mineralization technology
A magnesium sulfate subtype and natural evaporation technology, which is applied in the purification of alkali metal sulfite/sulfate, inorganic fertilizer, agriculture, etc., can solve the problem of large impact on langbeinite production, reduced sulfate content, and increased production costs, etc. problems, to achieve the effect of improving ore-forming efficiency, increasing the ratio of sulfur and potassium, and reducing the loss of brine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
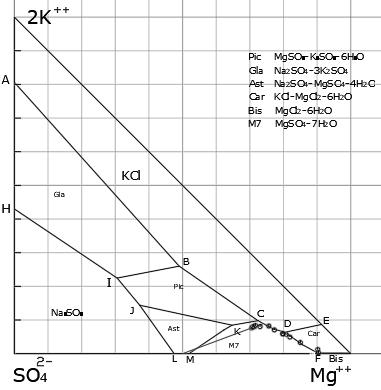
[0034] The raw material is taken from the underground brine brine pumping station in the Xitai Jinaier Salt Lake in Qaidam Basin, Qinghai in January 2018. It has the chemical composition shown in Table 1. From the composition analysis, the hydrochemical type of the brine system belongs to the magnesium sulfate subtype. Potassium ions composed of the brine Jenec J 2K =8.0, Mg Ion Jenec J Mg =68.81, sulfate ion Yenecke J SO4 =23.19, its brine composition point is located at 25℃K + 、Na + , Mg 2+ ∥ Cl - , SO 4 2- -H 2 The Epsom salt region in the metastable phase diagram of the O quinary water-salt system.
[0035] Table 1: Chemical composition of brine from wells in Xitaijinel Salt Lake in January 2018
[0036]
[0037] (1) Take 213.86 liters of brine from a magnesium sulfate subtype salt lake well, and use a plastic basin for natural evaporation to precipitate sodium chloride. + ] to 24.243g·L -1 , [Mg 2+ ] is 67.825g·L -1 , [Cl - ] is 194.580g·L -1 , [SO 4 2...
Embodiment 2
[0044] The raw materials are taken from the brine pumping station in April 2018 in Xitaijiner Salt Lake, Qaidam Basin, Qinghai. It has the chemical composition shown in Table 3. From the composition analysis, the hydrochemical type of the brine system belongs to the magnesium sulfate subtype. Potassium ions composed of the brine Jenec J 2K =7.07, magnesium ion Jenecke J Mg =74.12, sulfate ion Yenecke J SO4 =18.82, its brine composition point is located at 25℃K + 、Na + , Mg 2+ ∥ Cl - , SO 4 2- -H 2The Epsom salt region in the metastable phase diagram of the O quinary water-salt system. Compared with the method provided in Example 1, the main difference between this example is that the selected raw materials have different chemical compositions, and the adaptability of the method under different raw materials is mainly investigated.
[0045] Table 3: Chemical composition of brine from wells in Xitai Jinel Salt Lake in April 2018
[0046]
[0047] (1) Take 46.2 kg of...
Embodiment 3
[0054] The raw material is taken from the underground brine brine pumping station in Xitaijiner Salt Lake, Qaidam Basin, Qinghai in October 2018. It has the chemical composition shown in Table 5. From the composition analysis, the water chemical type of the brine system belongs to the subtype of magnesium sulfate . Potassium ions composed of the brine Jenec J 2K =7.68, magnesium ion Jenecke J Mg =76.26, sulfate ion Yenecke J SO4 =16.07, its brine composition point is located at 25℃K + 、Na + , Mg 2+ ∥ Cl - , SO 4 2- -H 2 The Epsom salt region in the metastable phase diagram of the O quinary water-salt system. Compared with the method provided in Example 2, the main difference between this example is that the selected raw materials have different chemical compositions, and the adaptability of the method under different raw materials is mainly investigated.
[0055] Table 5: Chemical composition of brine from wells in Xitai Jinel Salt Lake in October 2018
[0056]
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com