Method for preparing 2-chloro-1,1,1,4,4,4-hexafluoro-2-butene
A technology of butene and trifluorobutane, which is applied in the field of liquid-phase fluorination to prepare 2-chloro-1,1,1,4,4,4-hexafluoro-2-butene, which can solve the problem of poor product selectivity, Poor thermal stability, short life and other problems, achieve high reaction selectivity, improve reaction selectivity, and long catalyst life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
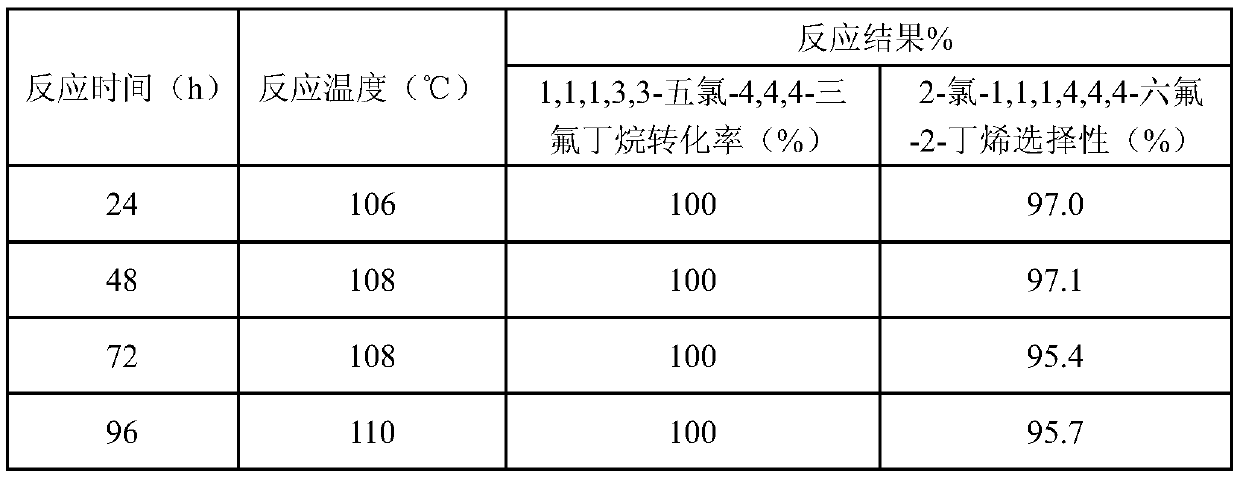
[0017] The preparation of 2-chloro-1,1,1,4,4,4-hexafluoro-2-butene by liquid phase fluorination was carried out in a stirred 250mL stainless steel autoclave. Put 12.4g TiF into the reactor in sequence 4 , 5.1g triethylamine, 12g HF and 5.96g 1,1,1,3,3-pentachloro-4,4,4-trifluorobutane, the reaction temperature is 120°C, and the reaction is 5h. After the reaction, the sample was washed with water to remove acid and analyzed by gas chromatography. The results showed that the conversion rate of 1,1,1,3,3-pentachloro-4,4,4-trifluorobutane was 100%, and 2-chloro-1 , The selectivity of 1,1,4,4,4-hexafluoro-2-butene was 96.5%.
Embodiment 2~14
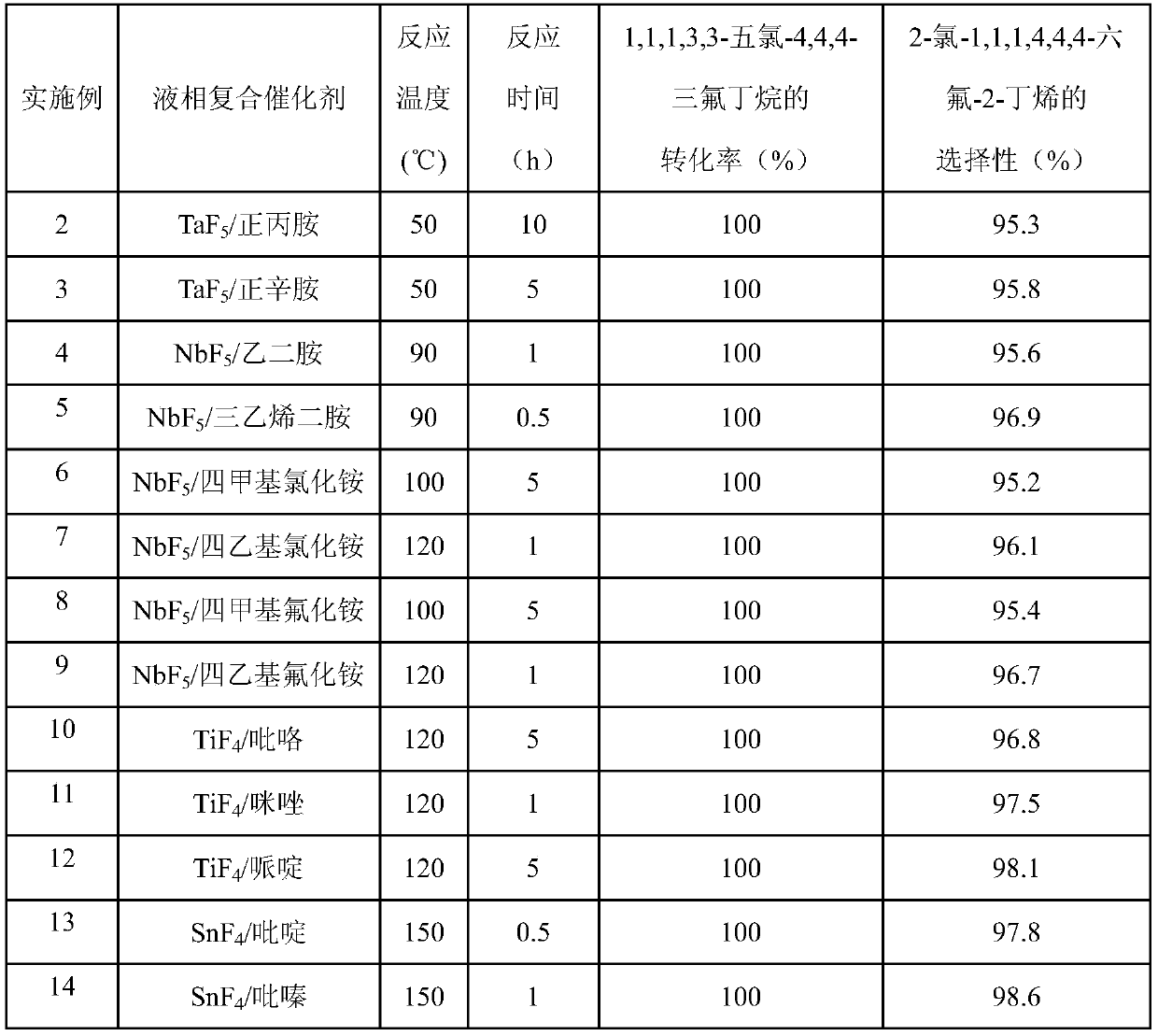
[0019] Examples 2-14 The preparation of 2-chloro-1,1,1,4,4,4-hexafluoro-2-butene by liquid phase fluorination reaction is the same as that of Example 1, except that the liquid phase composite catalyst group is changed Minutes, reaction temperature and reaction time, the reaction results are shown in Table 1.
[0020] Table 1
[0021]
Embodiment 15
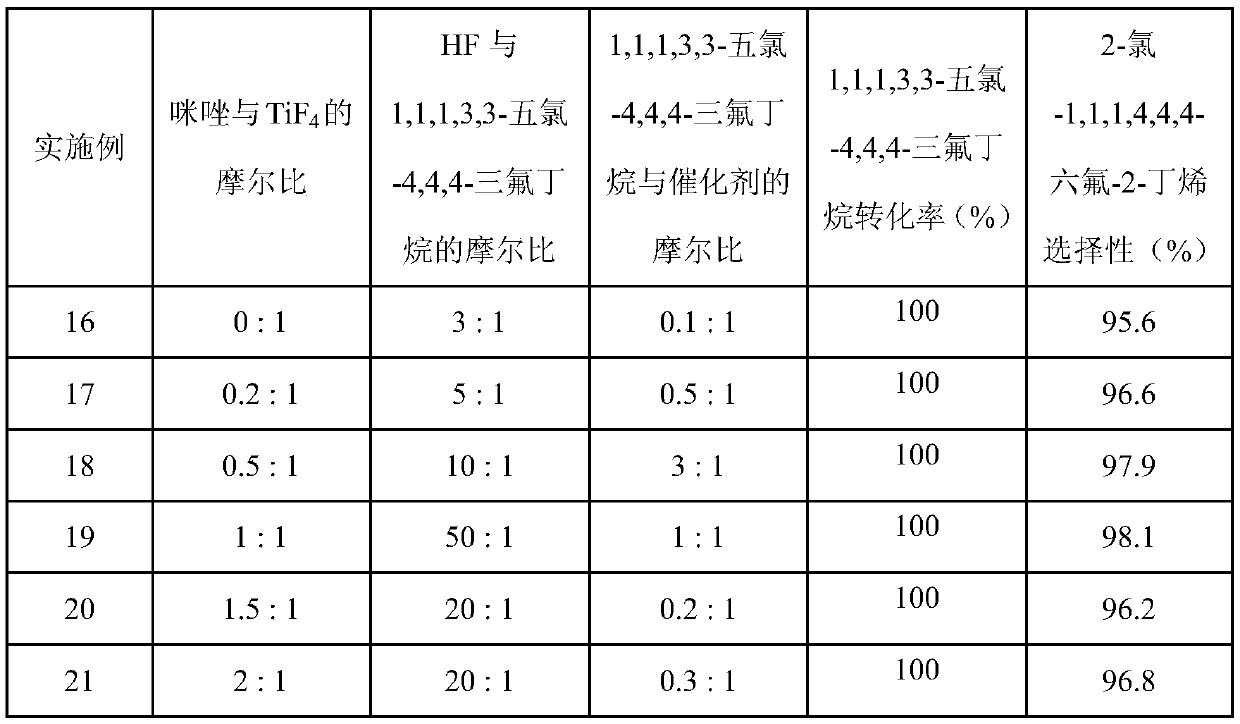
[0023] In the reactor identical with embodiment 1. Add 19.0g TiCl to the reactor in sequence 4 , and then add 60g HF to carry out fluorination treatment. During the treatment process, the generated HCl is eliminated through the gas phase port, and the pressure is controlled within 0.20MPa. Raise the temperature to 90°C, keep the temperature constant for 2 hours, and the treatment process ends. Add 5.96g of 1,1,1,3,3-pentachloro-4,4,4-trifluorobutane into the reaction kettle, the reaction temperature is 120°C, and cool down after 2 hours of reaction. After the sample was washed with water to remove acid, the gas chromatography analysis showed that the conversion rate of 1,1,1,3,3-pentachloro-4,4,4-trifluorobutane was 100%, and 2-chloro-1,1,1, The selectivity of 4,4,4-hexafluoro-2-butene was 98.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com