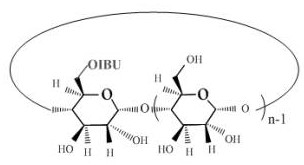
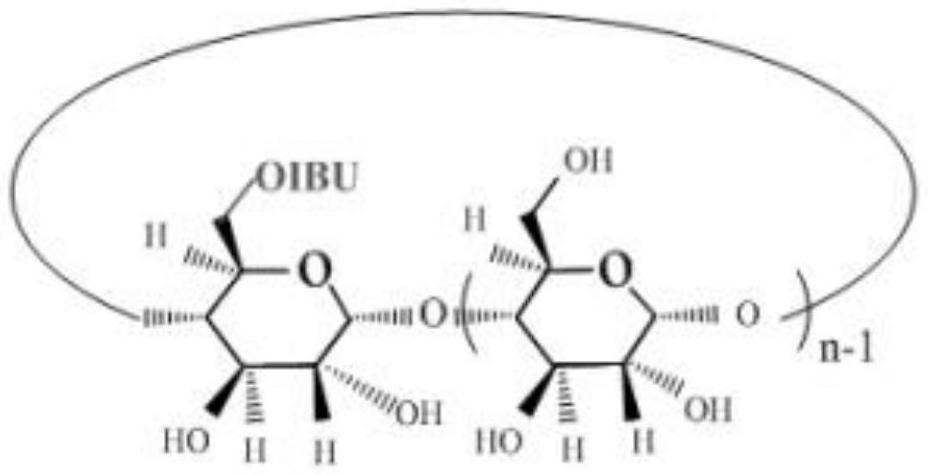
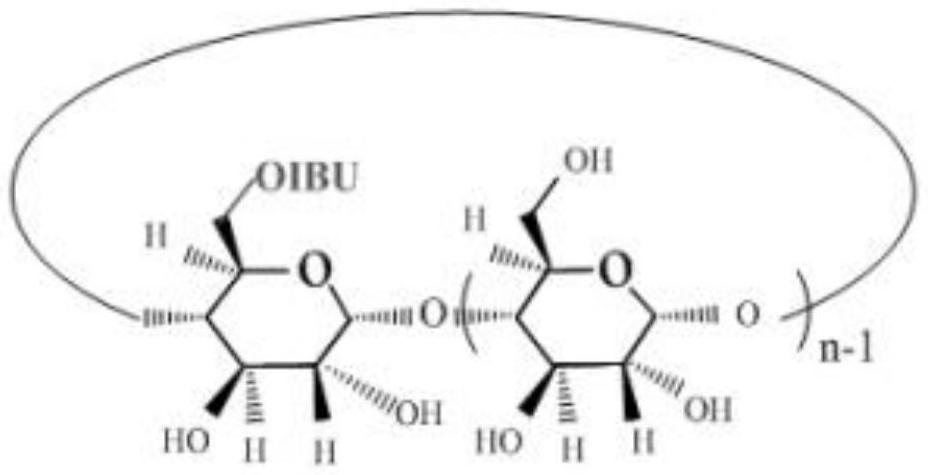
An antipyretic and analgesic ibuprofen-β-cyclodextrin first-side derivative and preparation method thereof
An antipyretic and analgesic, cyclodextrin technology, applied in antipyretics, drug combinations, pharmaceutical formulations, etc., can solve the problems of reducing ibuprofen antipyretic, analgesic time, hemolysis, and large fluctuations in blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] A preparation method of the antipyretic and analgesic ibuprofen-β-cyclodextrin first derivative as described in any one of claims 1-2, comprising the following steps:
[0026] Step a), the synthesis of ibuprofen imidazolate, ibuprofen is dissolved in dichloromethane, N, N'-carbonyldiimidazole (CDI) is dissolved in dichloromethane, and then the dichloromethane of ibuprofen The methane solution is added dropwise into the dichloromethane solution of CDI through the dropping funnel, stirred evenly until the reaction is completed, and dried after acid-base extraction and n-hexane precipitation to obtain imidazolate ibuprofen;
[0027] The synthetic route of ibuprofen imidazolate is:
[0028]
[0029]Step b), the synthesis of the first derivative of ibuprofen-β-cyclodextrin, the ibuprofen imidazolate prepared in step a) is dissolved in N,N-dimethylformamide (DMF), To form a DMF solution of imidazolate ibuprofen, add β-cyclodextrin to the DMF solution of imidazolate ibupro...
Embodiment 1
[0044] The preparation of ibuprofen imidazolate: ibuprofen 0.01mol (2.06g) is dissolved in 30ml dichloromethane; CDI0.015mol (2.43g) is dissolved in 60ml dichloromethane; The dichloromethane solution of ibuprofen is passed through The dropping funnel was added dropwise into the dichloromethane solution of CDI, reacted for 12-24 hours, extracted with acid and alkali, precipitated with n-hexane and dried to obtain imidazolate ibuprofen (yield 80%).
[0045] The ibuprofen imidazolate that this embodiment makes, nuclear magnetic data is as follows:
[0046] R f =0.53; 1 H NMR (400MHz, DMSO-d 6 ):8.49,7.71,7.32,7.30,7.13,7.11, 7.01,4.76,4.74,2.51,2.39,2.37,1.82-1.72,1.49,1.47,0.82,0.80; 13 C NMR (400MHz, DMSO-d 6 ): 171.8, 140.88, 137.63, 130.75, 130.07, 127.53, 117.24, 44.61, 44.36, 40.60-39.44, 29.99, 22.59, 19.59.
[0047] Preparation of ibuprofen β-cyclodextrin first side derivative: β-cyclodextrin (2.55 g, 2.25 mmol) was added into a 250 ml round bottom flask, and then 80...
Embodiment 2
[0050] Preparation of the first side derivative of ibuprofen β cyclodextrin: β-cyclodextrin (2.25g, 2.00mmol) was added in a 250ml round bottom flask, and other steps were the same as in Example 1, thereby obtaining purified ibuprofen The first side derivative of fen β-cyclodextrin (yield 28%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com