Hydrophilic gel sustained-release tablet containing echinacoside as well as preparation method and application thereof
A technology of echinacoside and hydrophilic gel, which is applied to medical preparations containing active ingredients, medical preparations without active ingredients, and pharmaceutical formulas, and can solve the problems of large daily doses, frequent takings, and safety issues. Low-level problems, to achieve the effect of reducing the frequency and dose of taking, reducing toxic and side effects, and enhancing compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
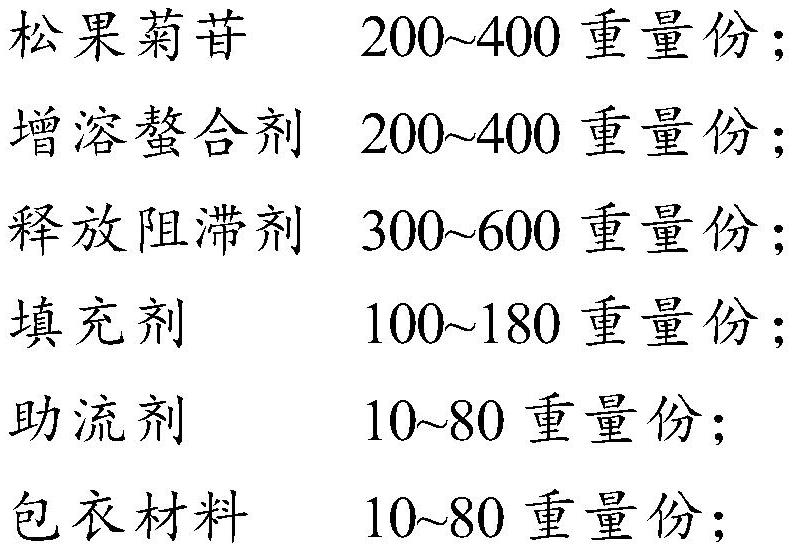
[0082] Preferably, the present invention provides a method for preparing the above-mentioned hydrophilic gel sustained-release tablet containing echinacoside, comprising the following steps:
[0083] The echinacoside and the solubilizing and chelating agent solution are mixed, filtered and dried to obtain the solubilizing and chelating agent inclusion complex of echinacoside.
[0084] The mass ratio of the echinacoside to the solubilizing and chelating agent is preferably 1:1.
[0085] Then the above inclusion compound is mixed with a release retardant and a first filler, and granulated.
[0086] Then, the granules obtained above are mixed with a glidant and a second filler, and compressed into tablets to obtain tablet cores.
[0087] The above-mentioned tablet core is coated to obtain the hydrophilic gel sustained-release tablet containing echinacoside.
[0088] The above-mentioned granulation is preferably wet granulation.
[0089] In some specific embodiments of the pres...
Embodiment 1
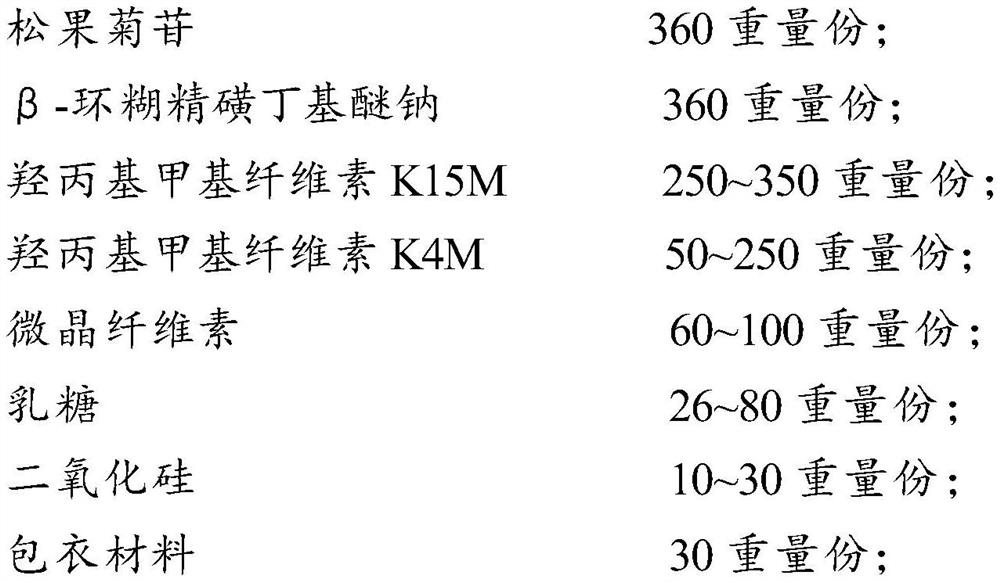
[0106] Prepare according to the dosage of prescription 1 in Table 1.
[0107] Preparation method: crush echinacoside to prepare chelate, sieve, fully mix with hydroxypropyl methylcellulose K15M, hydroxypropyl methylcellulose K4M, microcrystalline cellulose, make soft material, and granulate ;Dried, granulated, added lactose, silicon dioxide, mixed evenly, compressed into tablets, coated.
[0108] (1) Grind 360 parts by weight of echinacoside, pass through a 100-mesh pharmacopoeia sieve, weigh according to the prescription, mix with β-cyclodextrin sulfobutyl ether sodium solution in a ratio of 1:1, stir evenly, and filter , dried, and passed through a 100-mesh pharmacopoeia sieve to obtain echinacoside-β-cyclodextrin sulfobutyl ether sodium clathrate (that is, ECH-(SBE)7m-β-CD, 1:1), for subsequent use;
[0109] (2) Take each adjuvant according to the prescription ratio respectively, fully mix the raw material with the hydroxypropyl methylcellulose K15M of 250 parts by weight,...
Embodiment 2
[0115] Prepare according to the dosage of prescription 2 in table 1.
[0116] Preparation method: crush echinacoside to prepare chelate, sieve, fully mix with hydroxypropyl methylcellulose K15M, hydroxypropyl methylcellulose K4M, microcrystalline cellulose, make soft material, and granulate ;Dried, granulated, added lactose, silicon dioxide, mixed evenly, compressed into tablets, coated.
[0117] Concrete processing parameter is with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com