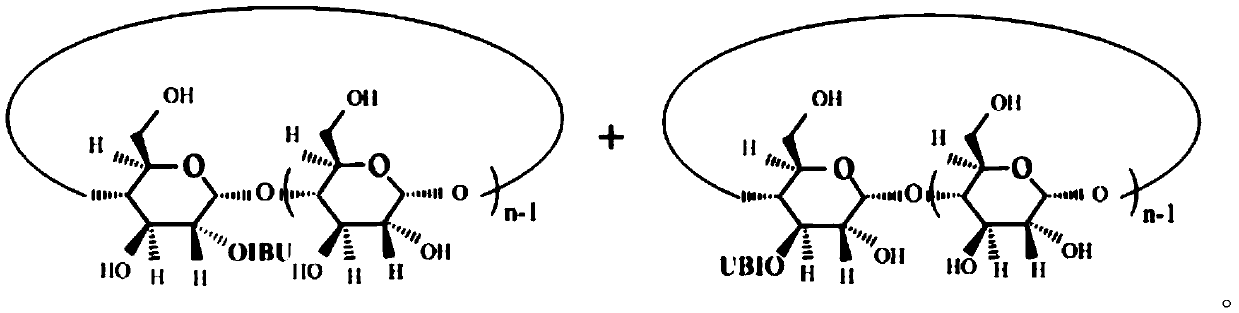
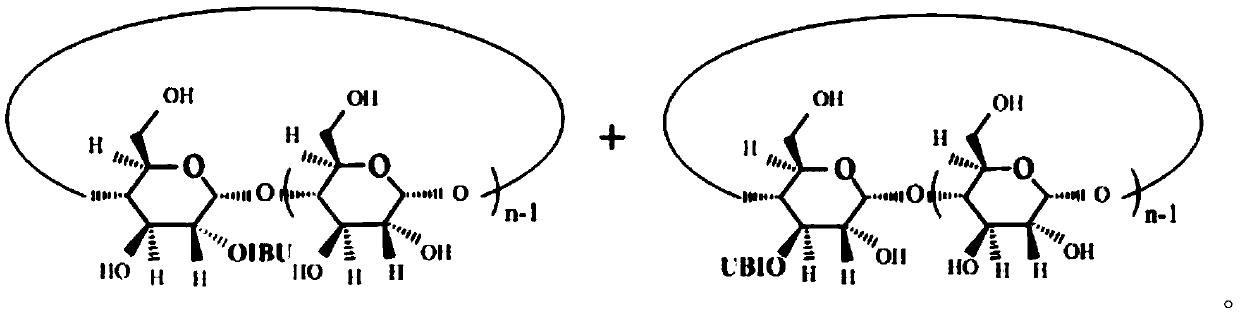
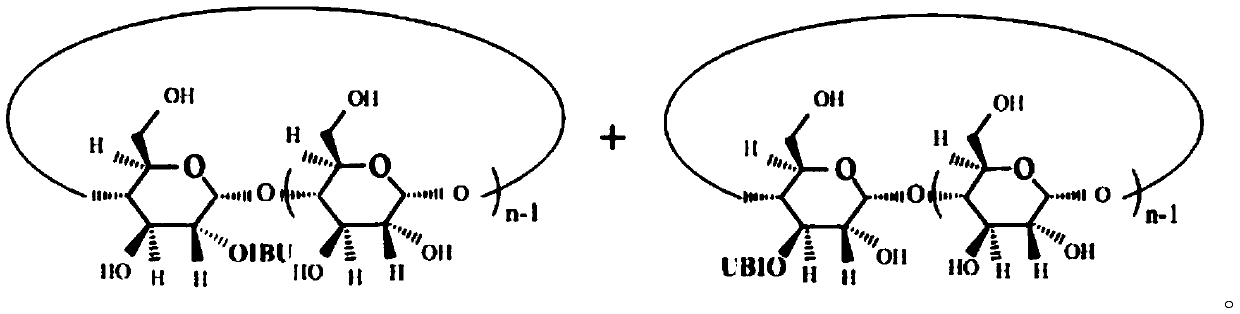Antipyretic and analgesic ibuprofen-beta-cyclodextrin secondary-side derivative and preparation method thereof
An antipyretic and analgesic, cyclodextrin technology, applied in the directions of antipyretics, drug combinations, pharmaceutical formulations, etc., can solve the problem of reducing the antipyretic and analgesic time of ibuprofen, the large fluctuation of blood drug concentration, and the aggravation of bleeding tendency. and other problems, to avoid the peaks and valleys of drug efficacy, reduce fluctuations in blood concentration, and avoid heartburn.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The present invention also proposes a preparation method for antipyretic and analgesic ibuprofen-β-cyclodextrin second derivatives, comprising the following steps:
[0024] Step a), the synthesis of ibuprofen imidazolate, ibuprofen is dissolved in dichloromethane, N, N'-carbonyldiimidazole (CDI) is dissolved in dichloromethane, and then the dichloromethane of ibuprofen The methane solution is added dropwise into the dichloromethane solution of CDI through the dropping funnel, stirred evenly until the reaction is completed, and dried after acid-base extraction and n-hexane precipitation to obtain imidazolate ibuprofen;
[0025] The synthetic route of ibuprofen imidazolate is:
[0026]
[0027] Step b), the synthesis of ibuprofen-β-cyclodextrin second side derivatives, the ibuprofen imidazolate prepared in step a) is dissolved in N,N-dimethylformamide (DMF), Add β-cyclodextrin to the DMF solution of ibuprofen imidazolate, stir evenly at room temperature, then add a ca...
Embodiment 1
[0041] The preparation of ibuprofen imidazolate: ibuprofen 0.01mol (2.06g) is dissolved in 30ml dichloromethane; CDI0.015mol (2.43g) is dissolved in 60ml dichloromethane; The dichloromethane solution of ibuprofen is passed through The dropping funnel was added dropwise into the dichloromethane solution of CDI, reacted for 12-24 hours, extracted with acid and alkali, precipitated with n-hexane and dried to obtain imidazolate ibuprofen (yield 80%).
[0042] The ibuprofen imidazolate that this embodiment makes, nuclear magnetic data is as follows:
[0043] 1 H NMR (400MHz, DMSO-d 6 ):8.49,7.71,7.32,7.30,7.13,7.11,7.01, 4.76,4.74,2.51,2.39,2.37,1.82-1.72,1.49,1.47,0.82,0.80; 13 C NMR (400MHz, DMSO-d 6 ): 171.8, 140.88, 137.63, 130.75, 130.07, 127.53, 117.24, 44.61, 44.36, 40.60-39.44, 29.99, 22.59, 19.59.
[0044] Preparation of ibuprofen β-cyclodextrin second side derivatives: Ibuprofen imidazolate 0.015mol (3.84g) was dissolved in 600mL N,N-dimethylformamide (DMF), and β-cyc...
Embodiment 2
[0047] The preparation of ibuprofen imidazolate: ibuprofen 0.01mol (2.06g) is dissolved in 30ml dichloromethane; CDI0.015mol (2.43g) is dissolved in 60ml dichloromethane; The dichloromethane solution of ibuprofen is passed through The dropping funnel was added dropwise into the dichloromethane solution of CDI, reacted for 5-10 hours, extracted with acid and alkali, precipitated with n-hexane and dried to obtain imidazolate ibuprofen (yield 68%).
[0048] Preparation of ibuprofen β-cyclodextrin second side derivatives: Ibuprofen imidazolate 0.015mol (3.84g) was dissolved in 600mL N, in N-dimethylformamide (DMF), added β-CD0. 0225mol (25.5g), while adding triethylamine (65ml). Continue to stir the reaction at room temperature for 12-24h, and evaporate the solvent under reduced pressure. The residue was chromatographed with excess acetone and filtered to obtain a crude product. The crude product was purified by a C18 reverse-phase column, and dried in vacuum to obtain a pure se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com