Method for producing low chroma polythiol compound
A polythiol and compound technology, which is applied in the preparation of thioether, organic chemistry, etc., can solve the problems of chromaticity oligomeric thiol compounds, yellowing of optical resin lenses, poor chromaticity of thiol compounds, etc., and achieve low dosage , reducing the effect of chroma, large social and economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
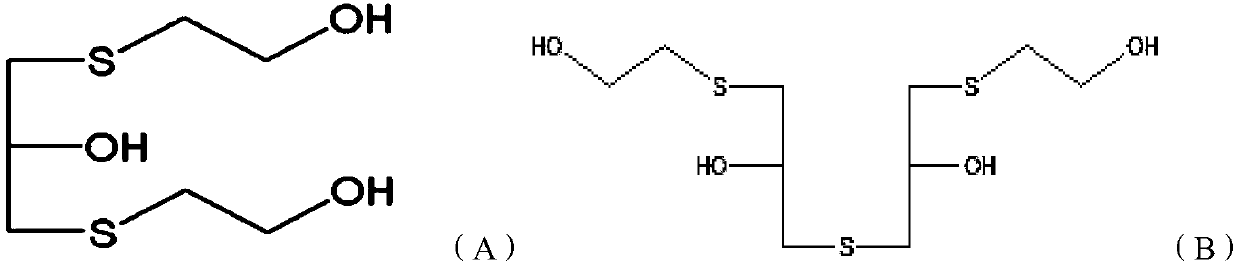
[0024] (a) Pour 190g of polyol A into a four-necked flask, add 115g (3mol) of thiourea and 152g of hydrochloric acid (3mol) with a mass fraction of 36%, and react at reflux at 100°C for 3.5h to form isothiuronium salt;
[0025] (b) After cooling down to room temperature, add sodium carbonate solution to adjust the pH of the system to 12, add 0.52 g of lauric acid at the same time, heat to 30 ° C, and carry out hydrolysis reaction for 2 hours to obtain a crude polythiol mixture;
[0026] (c) Mix and separate the crude product obtained in step (b), wash with water twice, dry and filter with 5% silica gel to obtain polythiol compound with low chroma.
[0027] The chromaticity of the polythiol product obtained in Example 1 of the present invention was detected by a high-precision multifunctional spectrophotometer, and the results showed that the chromaticity of the polythiol compound obtained in Example 1 of the present invention was 10 Hazen.
Embodiment 2
[0029] (a) Pour 200g of polyol B into a four-necked flask, add 123g of thiourea (3.2mol) and 177g of hydrochloric acid (3.5mol) with a mass fraction of 36%, and react at reflux at 110°C for 2h to form isothiourea salt;
[0030] (b) After cooling down to room temperature, add ammonia solution to adjust the pH of the system to 13, add 4g of sodium lauryl sulfate at the same time, heat to 45°C, and perform a hydrolysis reaction for 2.5 hours to obtain a crude polythiol mixture;
[0031] (c) mixing and separating the crude product obtained in step (b), washing with water twice, drying and filtering with 6% phosphorus pentoxide to obtain a polythiol compound with low chroma.
[0032] The chromaticity of the polythiol product obtained in Example 2 of the present invention was detected by a high-precision multifunctional spectrophotometer, and the results showed that the chromaticity of the polythiol compound obtained in Example 2 of the present invention was 9 Hazen.
Embodiment 3
[0034] (a) Pour 180g of polyol A into a four-necked flask, add 126g of thiourea (3.3mol) and 203g of hydrochloric acid (4mol) with a mass fraction of 36%, and react at reflux at 112°C for 2.5h to form isothiuronium salt;
[0035] (b) After cooling down to room temperature, add sodium hydroxide solution to adjust the pH of the system to 10, add 2 g of sodium dodecylbenzenesulfonate at the same time, heat to 70 ° C, and perform hydrolysis for 3.5 hours to obtain a crude polythiol mixture;
[0036] (c) mixing and separating the crude product obtained in step (b), washing with water twice, drying and filtering with 10% calcium oxide to obtain a polythiol compound with low chroma.
[0037] The chromaticity of the polythiol product obtained in Example 3 of the present invention was detected by a high-precision multifunctional spectrophotometer, and the results showed that the chromaticity of the polythiol compound obtained in Example 3 of the present invention was 8 Hazen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com