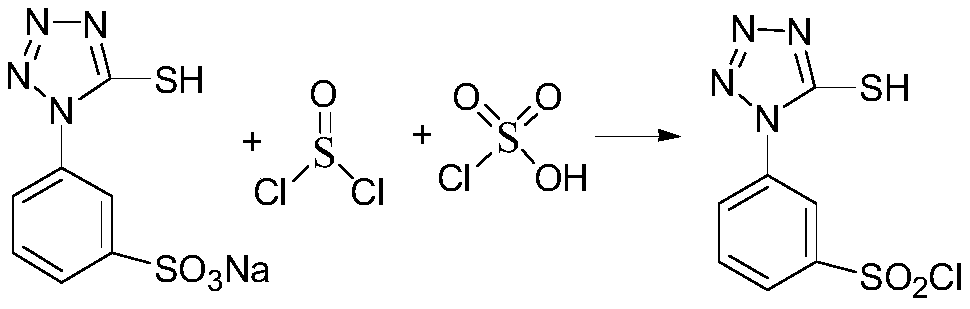
Preparation method of 3-(5-mercapto-1-tetrazolyl)benzene sulfonyl chloride
A technology of benzenesulfonyl chloride and tetrazolyl, which is applied in the field of preparation of 3-benzenesulfonyl chloride, can solve the problem of increasing the difficulty of chlorination of sodium 3-(5-mercapto-1-tetrazolyl)benzenesulfonate and increasing the process Operation cost and sewage cost, influence of benzene ring sulfonylation activity, etc., to achieve the effect of improved yield, mild reaction conditions, and optimized reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
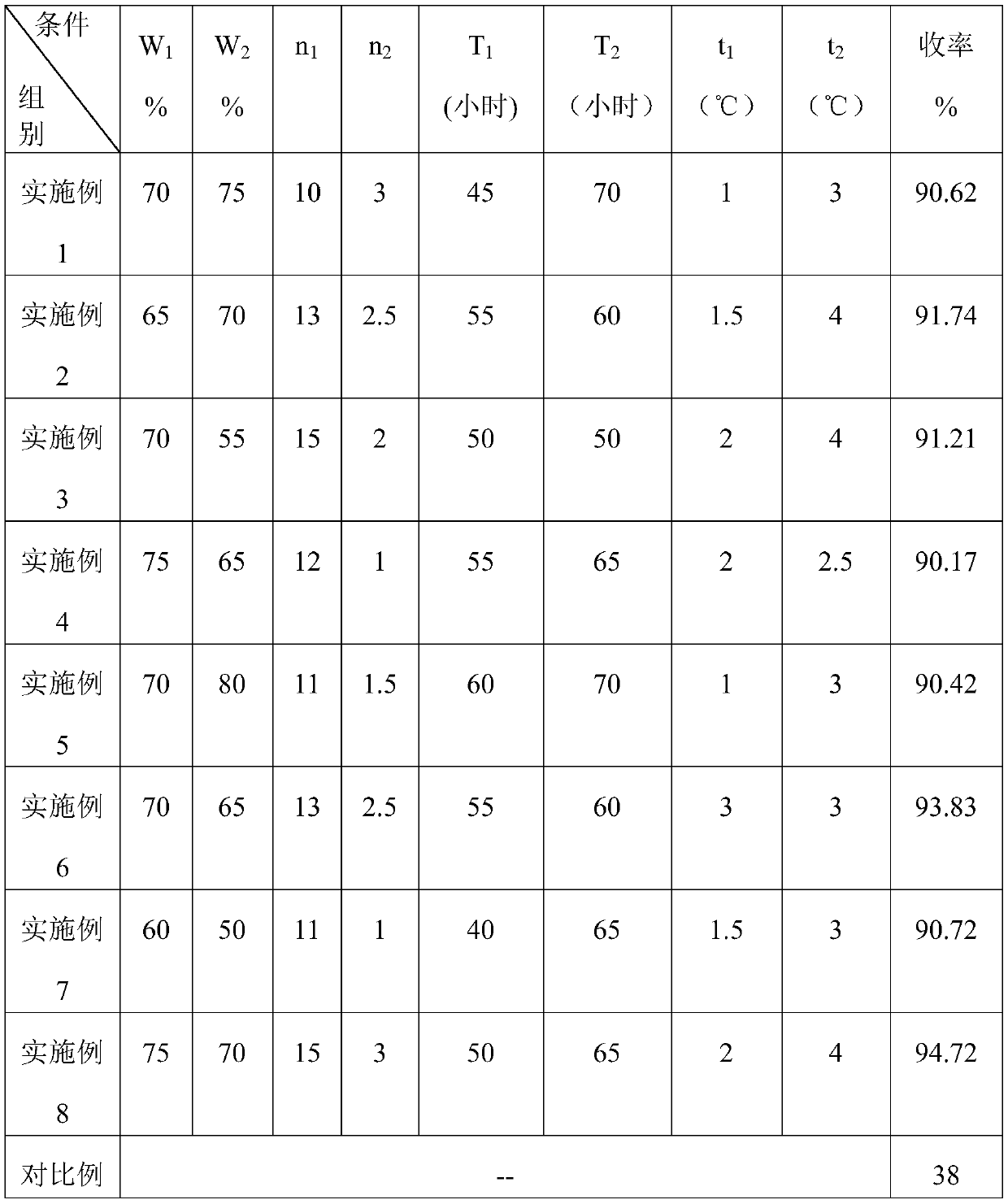
[0025] In a 100 mL three-necked flask, add 12 mL of chlorosulfonic acid, add 7.2 g of a mixture of sodium 3-(5-mercapto-1-tetrazolyl)benzenesulfonate (70%) and sodium chloride (30%) in batches and stir until solid Completely dissolve, add 3.9mL of thionyl chloride and catalytic amount of DMF dropwise at 45°C, drop it for 1h, raise the temperature to 70°C for 3hrs, add sulfuric acid with a mass concentration of 75% dropwise, and pour the reaction solution into the A white solid was precipitated in water, filtered and air-dried to obtain 4.51 g of 3-(5-mercapto-1-tetrazolyl)benzenesulfonyl chloride with a yield of 90.62%.
[0026] 1 HNMR (400MHz, (CD 3 )2CO)8.55(s,1H),8.42–8.44(d,1H)8.13(s,1H),8.13–8.17(t,1H)
Embodiment 2
[0028] In a 100 mL three-necked flask, add 25 mL of chlorosulfonic acid, add 12.4 g of a mixture of 3-(5-mercapto-1-tetrazolyl) benzenesulfonate sodium (65%) and sodium chloride (35%) in batches and stir until solid Completely dissolve, add 5.2mL of thionyl chloride and catalytic amount of DMF dropwise at 55°C, drop it for 1.5h, raise the temperature to 60°C and react for 4hrs, add sulfuric acid with a mass concentration of 70% dropwise, and pour the reaction solution until almost no bubbles are formed. A white solid was precipitated in water, filtered, and air-dried to obtain 7.30 g of 3-(5-mercapto-1-tetrazolyl)benzenesulfonyl chloride, with a yield of 91.74%.
Embodiment 3
[0030] In a 250 mL three-neck flask, add 53 mL of chlorosulfonic acid, add 21.4 g of a mixture of 3-(5-mercapto-1-tetrazolyl) benzenesulfonate sodium (70%) and sodium chloride (30%) in batches and stir until solid Completely dissolve, add 7.8mL of thionyl chloride and catalytic amount of DMF dropwise at 50°C, finish dropping for 2 hours, react at 50°C for 4hrs, add sulfuric acid with a mass concentration of 55% dropwise, and pour the reaction solution into water to precipitate after almost no bubbles are formed. The white solid was filtered and air-dried to obtain 13.49 g of 3-(5-mercapto-1-tetrazolyl)benzenesulfonyl chloride with a yield of 91.21%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com