Prostate cancer PET diagnostic reagent 68Ga-NOTA-ANCP-PSMA and preparation method and application thereof
A technology of NOTA-ANCP-PSMA, 68ga-nota-ancp-psma, applied in the preparation methods of peptides, chemical instruments and methods, preparations for in vivo tests, etc. protection and other issues, to achieve the effect of fast marking speed, high marking rate and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] This embodiment provides a prostate cancer PET diagnostic reagent 68 The preparation method of Ga-NOTA-ANCP-PSMA, described method comprises the steps:
[0042] (S1) The precursor compound NOTA-ANCP-PSMA is synthesized by solid phase synthesis;
[0043] Preparation of compound 3
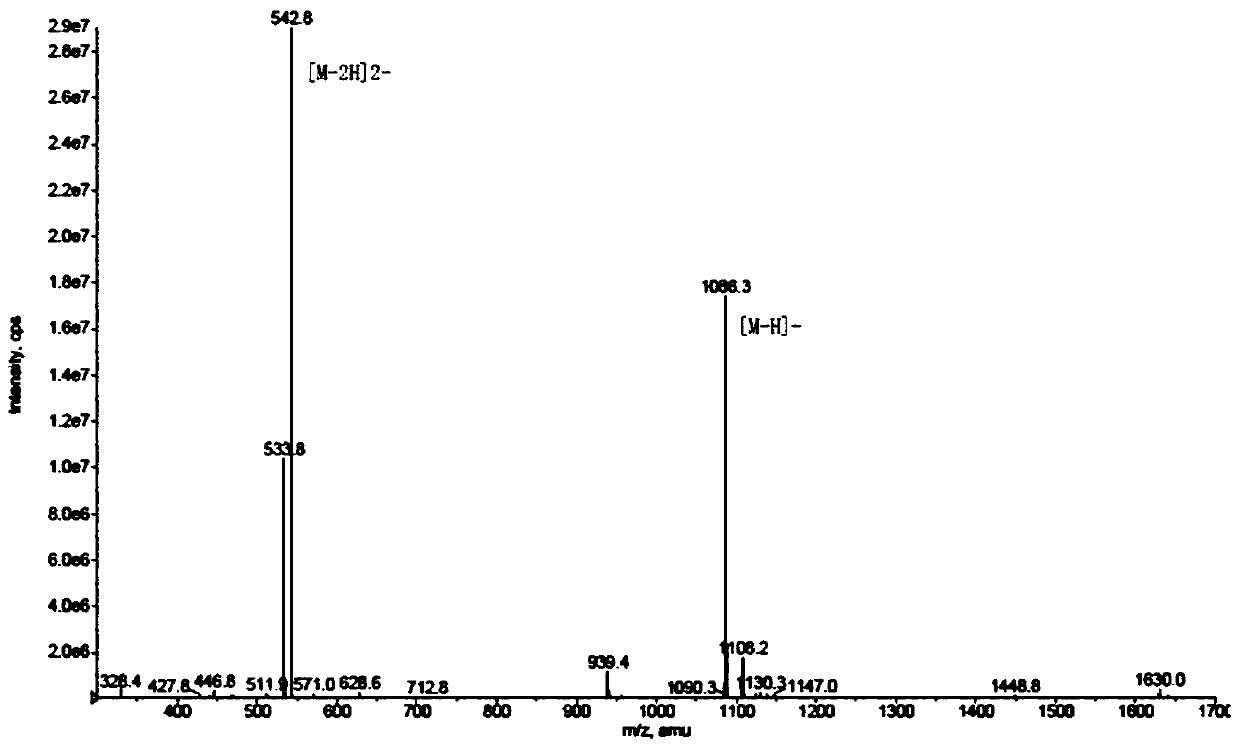
[0044] The synthetic route is shown below. Using Fmoc-Lys(Dde)-Wang Resin (compound 1) as the starting material, weigh 3g of the raw material with a substitution degree of 0.3mmol / g, add it to the reactor, add DMF, and soak for 30min. Then drain the DMF, add 3 times the volume of 20% Pip / DMF, and fill with nitrogen gas to remove Fmoc, react for 30 minutes, drain the 20% Pip / DMF, wash with DMF 5 times, and the ninhydrin detection shows dark blue. Add N,N'-succinimidyl carbonate (DSC), N,N-diisopropylethylamine (DIPEA) and 4-dimethylaminopyridine (DMAP) in proportion, and the input ratio is resin:DSC: DIPEA:DMAP=1:6:12:1, add an appropriate amount of DMF, and react for 1 h under nitrogen prot...
Embodiment 2
[0059] In vitro stability assay:
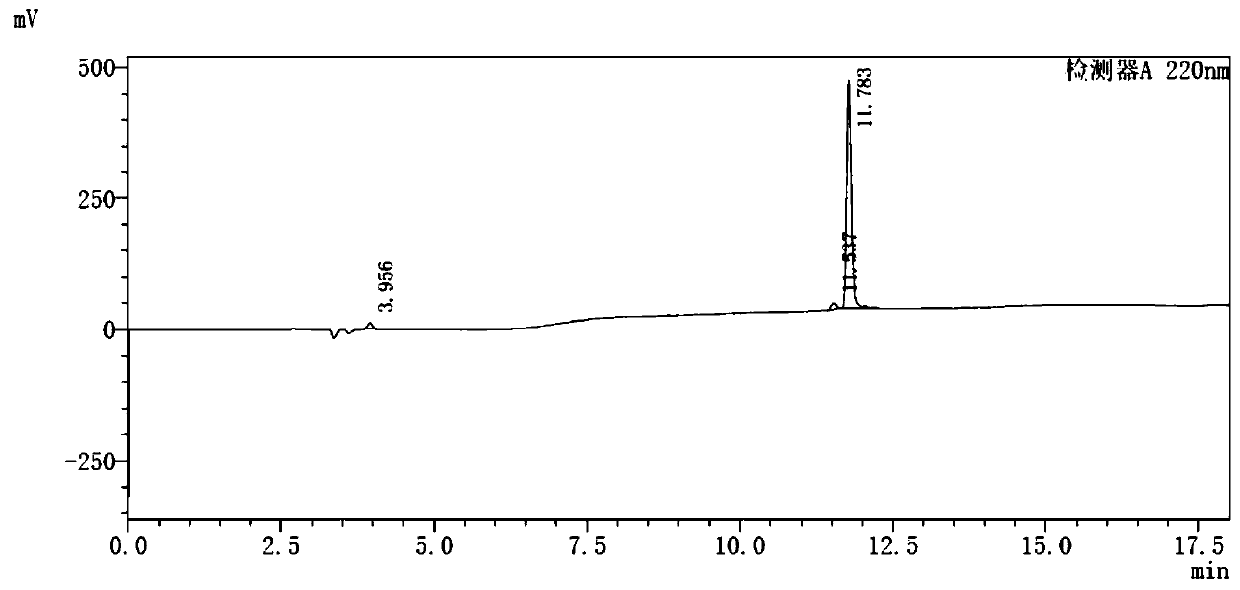
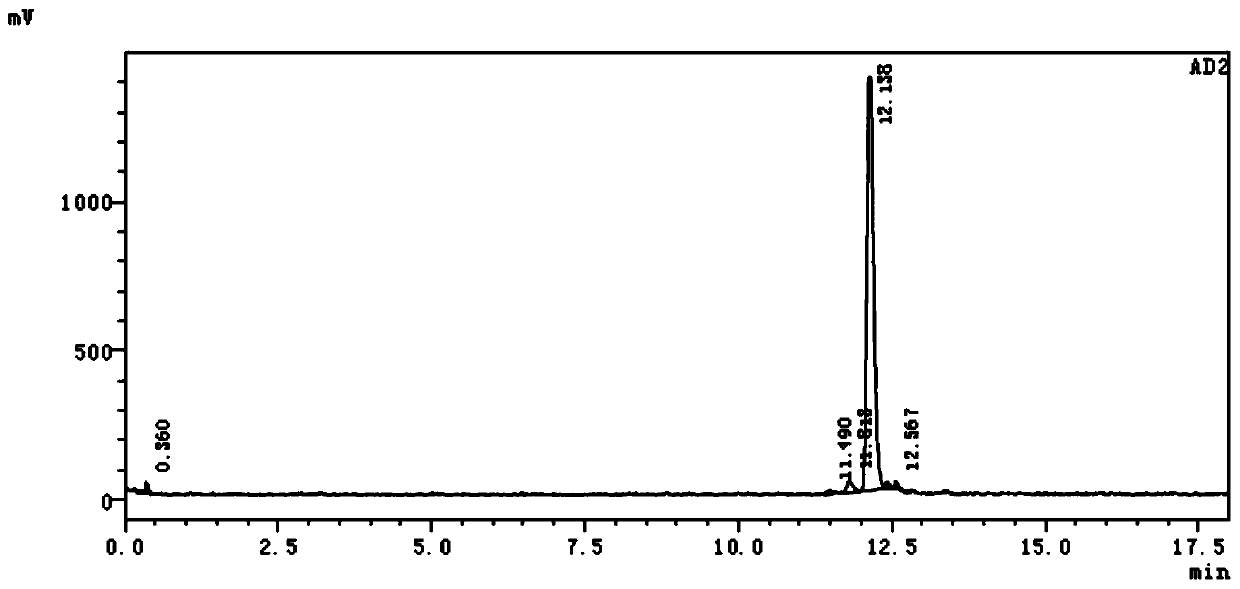
[0060] 68 The radiochemical stability of Ga-NOTA-ANCP-PSMA was carried out in two systems of calf serum and phosphate buffer. PBS method: place in 0.5mL phosphate buffer (PBS, pH=7.4), place at 37°C, incubate for 30, 60, 90, 120, 150min, then use HPLC to measure its radiochemical purity to determine its in vitro stability. Serum method: place in 0.5mL calf serum solution, incubate at pH=7, 37°C. When incubated for 30, 60, 90, 120, and 150 min, the radiochemical purity was determined by HPLC to determine its in vitro stability.
[0061] Such as Figure 4 as shown, 68 The stability of Ga-NOTA-ANCP-PSMA in PBS system was good, and the radiochemical purity remained above 95.4% until 150min. The in vitro stability results in the BSA system showed that the radiochemical purity could be maintained at a level above 95% within 90 minutes, and then decreased slightly. illustrate 68 The stability of Ga-NOTA-ANCP-PSMA in BSA is poorer than that i...
Embodiment 3
[0063] Measurement of lipid-water partition coefficient:
[0064] Mark 68 Ga-NOTA-ANCP-PSMA was separated and purified by Sep-Pak C18 Cartridge, and eluted with 95% ethanol. The obtained marker was blown dry by dry nitrogen, and the obtained marker was dissolved in a 1.5mL EP tube (about 3.7MBq) using the same volume (0.5mL: 0.5mL) of n-octanol and phosphate buffer solution (pH=7.4) middle. Fully shake for 5 minutes, and centrifuge the layers in a centrifuge for 5 minutes at a speed of 2000 rpm. Take 100uL each of the organic phase and the aqueous phase in 1mL EP tubes, measure their radioactive counts in the well-type γ detector, and calculate the lipid-water partition coefficient P from the ratio of the radioactive counts of the organic phase and the aqueous phase. P=log(N o / N W )(N o and N W are the counts of the organic and aqueous phase samples, respectively). The operation was repeated 3 times, and the average value was taken as the lipid-water partition coeffic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com