A kind of niraparib granule efficiently utilized and preparation method thereof
A kind of granule and high-efficiency technology, which is applied in the field of high-efficiency niraparib granule and its preparation, can solve the problems of short service life and inconvenient application of electronically controlled valves, and achieve the effect of maintaining drug effect, increasing concentration and reducing content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
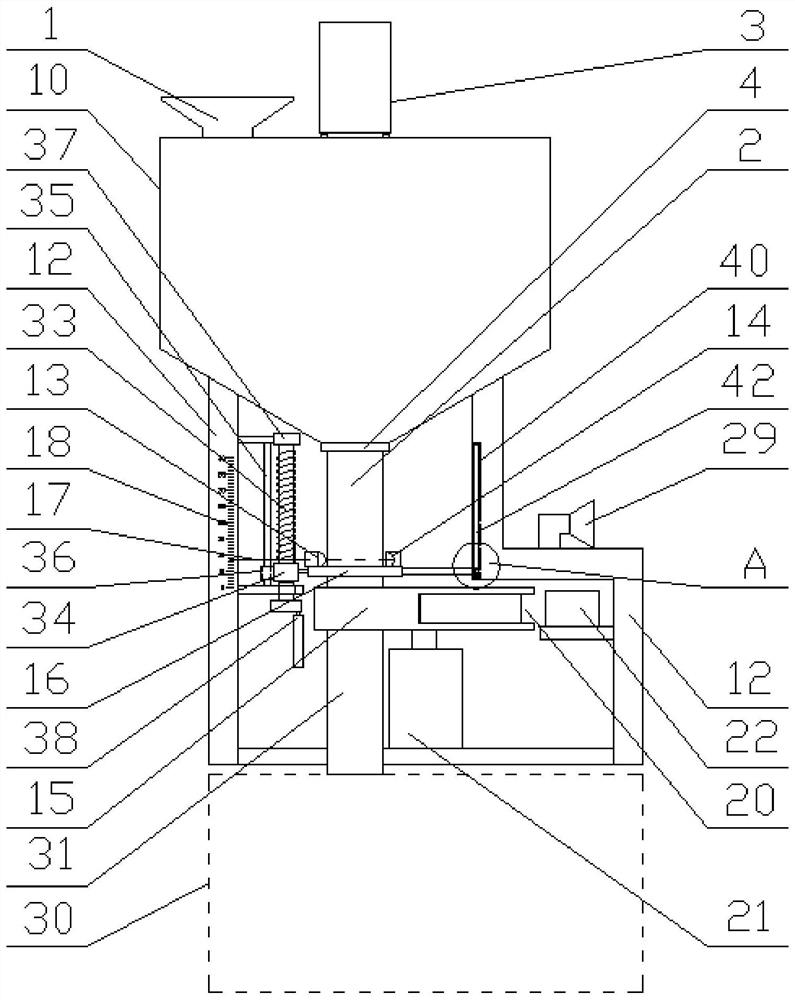
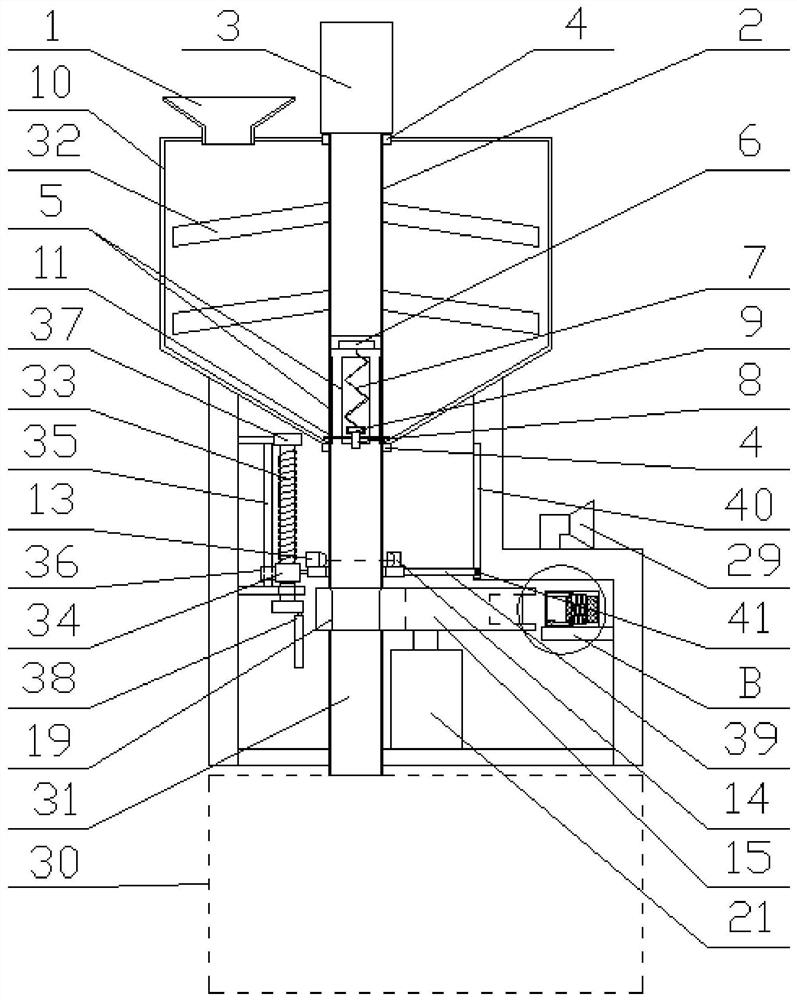
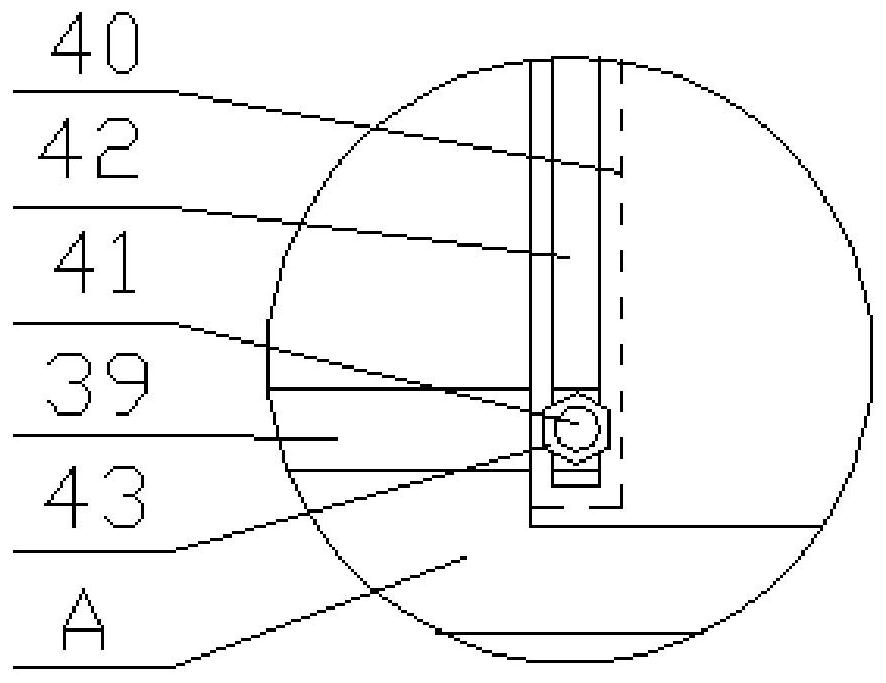
Image
Examples
Embodiment 1
[0051] A highly efficient niraparib granule, comprising the following components in parts by weight:
[0052] 5 parts nirapani;
[0053] 5 parts of carboxylesterase inhibitors;
[0054] 8 parts of glucose;
[0055] Vitamin C 2 parts;
[0056] 75 parts of filler;
[0057] 3 parts of disintegrant.
[0058] In this embodiment, the filler is pregelatinized starch.
[0059] In this embodiment, the disintegrating agent is a composition of sodium carboxymethyl starch, low-substituted hydroxypropyl cellulose and croscarmellose sodium, and their mass ratio is 2:1:1.
[0060] The preparation method of the niraparib granule of above-mentioned efficient utilization comprises the steps:
[0061] S1. Soft material preparation: After making a thick paste from highly utilized niraparib granules, carboxylesterase inhibitors, glucose and vitamin C, mix it with a filler and a disintegrant to make a soft material;
[0062] S2. Making wet granules: turn on the swing granulator to preheat, a...
Embodiment 2
[0082] The difference between this embodiment and embodiment 1 is:
[0083] A highly efficient niraparib granule, comprising the following components in parts by weight:
[0084] Nirapani 6.5 parts;
[0085] 4.5 parts of carboxylesterase inhibitors;
[0086] Glucose 8.5 parts;
[0087] Vitamin C 2.5 parts;
[0088] 72 parts of filler;
[0089] 3.5 parts of disintegrant.
[0090] In this embodiment, the filler is a composition of lactose and microcrystalline cellulose, and their mass ratio is 2:5.
[0091] In this embodiment, the disintegrant is low-substituted hydroxypropyl cellulose.
[0092] In this embodiment, the mesh size of the oscillating granulator described in step S2 of the preparation method is 50 mesh, and the rotating speed of the drum of the oscillating granulator is 5 r / min.
[0093] In the present embodiment, according to claim 1, a highly efficient Niraparib granule is characterized in that the temperature of the hot air introduced in the boiling dryer ...
Embodiment 3
[0096] The difference between this embodiment and embodiment 1 is:
[0097] A highly efficient niraparib granule, comprising the following components in parts by weight:
[0098] Nirapani 7.5 parts;
[0099] 4 parts of carboxylesterase inhibitor;
[0100] 9 parts of glucose;
[0101] Vitamin C 3 parts;
[0102] 70 parts of filler;
[0103] 4 parts of disintegrant.
[0104] In this embodiment, the filler is a composition of pregelatinized starch, lactose and microcrystalline cellulose, and their mass ratio is 3:1:2.
[0105] In this embodiment, the disintegrating agent is a composition of sodium carboxymethyl starch and croscarmellose sodium, and their mass ratio is 3:1.
[0106] In this embodiment, the mesh size of the oscillating granulator described in step S2 of the preparation method is 50 mesh, and the rotating speed of the drum of the oscillating granulator is 80 r / min.
[0107] In the present embodiment, according to claim 1, a highly efficient Niraparib granule is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com