Catalyst to catalytically degrade formaldehyde at normal temperature and preparation method and application thereof
A catalytic degradation and catalyst technology, which is applied in the field of catalyst materials for purifying formaldehyde, can solve the problems of unstable activity, large specific surface area, and high preparation cost, and achieve good removal effect, simple process conditions, and optimized structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] A preparation method of a catalyst for catalyzing formaldehyde at normal temperature, comprising the following steps:
[0071] (1) Take 2.864g 50% Mn(NO 3 ) 2 solution, 9.114g Ce(NO 3 ) 3 ·6H 2 O, dissolved in 100mL water, while 4.582g KMnO 4 dissolved therein to obtain a reaction solution, which was placed on a magnetic stirrer and stirred for 2 h.
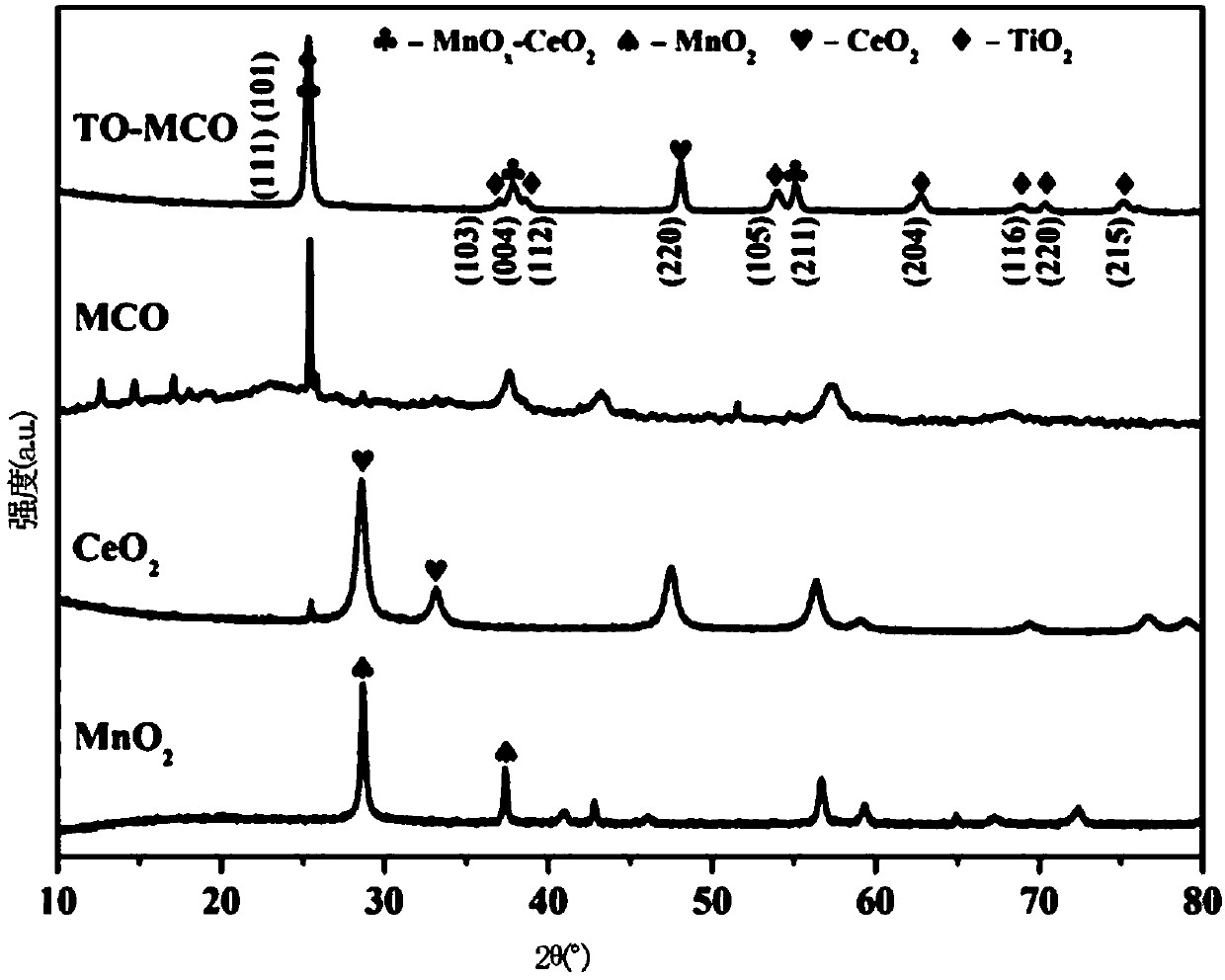
[0072] (2) Use 1mol / L NH 3 ·H 2 O adjust the pH of the solution obtained in step (1) to about 8.0, then move the reaction solution into a three-necked flask, and start the reflux condensation reaction in an oil bath at 60°C; % TiO 2 sol, the TiO 2 Sol, TiO 2 The concentration is 0.020g / mL, stir evenly, close the bottle mouth, reflux condensation reaction lasts for 4h; centrifuge the precipitate and wash until the supernatant is clear, take the centrifuged precipitate and fully dry it at 70°C, transfer it to the muffle furnace Calcined at 350°C for 5h to obtain TiO 2 load MnO x -CeO 2 (abbreviation: TO-MCO) ca...
Embodiment 2
[0079] A preparation method of a catalyst for catalyzing formaldehyde at normal temperature, comprising the following steps:
[0080] (1) Take 6.263g of 50% Mn(NO 3 ) 2 solution, 21.711g Ce(NO 3 ) 3 ·6H 2 O, dissolved in 500mL water, while 10.667g KMnO 4 dissolved therein to obtain a reaction solution, which was placed on a magnetic stirrer and stirred for 2 h.
[0081] (2) Use 1mol / L NH 3 ·H 2 O adjust the pH of the solution obtained in step (1) to about 8.0, then move the reaction solution into a three-necked flask, and start the reflux condensation reaction in an oil bath at 60°C; %TiO 2 sol, the TiO 2 Sol, TiO 2 The concentration is 0.020g / mL, stir evenly, close the bottle mouth, reflux condensation reaction lasts for 4h; centrifuge the precipitate and wash until the supernatant is clear, take the centrifuged precipitate and fully dry it at 70°C, transfer it to the muffle furnace Calcined at 360°C for 5h to obtain TiO 2 load MnO x -CeO 2 (abbreviation: TO-MCO...
Embodiment 3
[0083] A preparation method of a catalyst for catalyzing formaldehyde at normal temperature, comprising the following steps:
[0084] (1) Take 6.800g of 50% Mn(NO 3 ) 2 solution, 21.711g Ce(NO 3 ) 3 ·6H 2 O, dissolved in 600mL water, while 10.904g KMnO 4 dissolved therein to obtain a reaction solution, which was placed on a magnetic stirrer and stirred for 2 h.
[0085] (2) Use 1mol / L NH 3 ·H 2 O adjust the pH of the solution obtained in step (1) to about 8.0, then move the reaction solution into a three-necked flask, and start the reflux condensation reaction in an oil bath at 50°C; %TiO 2 sol, the TiO 2 Sol, TiO 2 The concentration is 0.020g / mL, stir evenly, close the bottle mouth, reflux condensation reaction lasts for 4h; centrifuge the precipitate and wash until the supernatant is clear, take the centrifuged precipitate and fully dry it at 70°C, transfer it to the muffle furnace Calcined at 320°C for 5h to obtain TiO 2 load MnO x -CeO 2 (abbreviation: TO-MCO...
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
| radius | aaaaa | aaaaa |
| radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com