High-concentration organic saline solution-based method for preparing graphene through electrochemical intercalation
An organic salt and aqueous solution technology, which is applied in the field of electrochemical intercalation preparation of graphene based on high-concentration organic salt aqueous solution, can solve the problems of affecting the crystal structure of graphene, uncontrollable number of graphene layers, destruction of graphite electrodes, etc., and achieves the preparation cycle. Short, continuous controllable intercalation, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
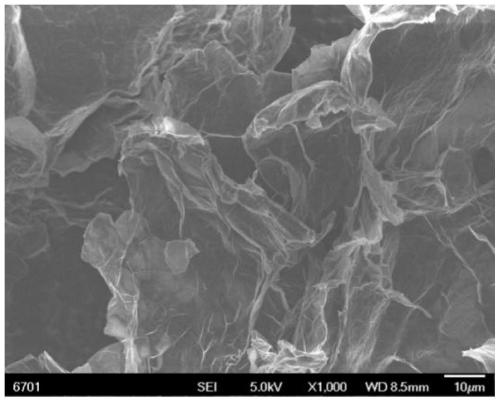
[0038] Using lithium bistrifluoromethanesulfonylimide as the electrolyte, weigh 18.09g of lithium bistrifluoromethanesulfonylimide, add 3g of water (the mass concentration of the organic salt is 85.8%), place the solution in an oven at 50°C for 30min, and wait for The solution is clear and transparent as electrolyte for electrochemical intercalation of graphite. Add the prepared electrolyte solution into the electrolytic cell, choose graphite paper as the anode and connect it to the DC power supply, and use the platinum sheet as the cathode to connect it to the DC power supply. The distance between the two electrodes is 5cm, apply a voltage of 20V, and intercalate and expand at 25°C The electrode can last for 3 hours without peeling off, and graphene aggregates are obtained, and the actual picture is as follows figure 1 As shown, after vacuum filtration and cleaning, the graphene dispersion was obtained by dispersing in an 80W ultrasonic cleaning machine for 30 minutes, and th...
Embodiment 2
[0041] Using lithium bistrifluoromethanesulfonimide as the electrolyte, weigh 5 mL of lithium bistrifluoromethanesulfonimide and add 1 mL of water (the volume concentration of the organic salt is 83.3%), and place the solution in an oven at 50°C for 30 minutes, and wait for The solution is clear and transparent as a high-concentration brine electrolyte for electrochemical intercalation of graphite. Add the prepared electrolyte in the electrolytic cell, choose graphite paper as the anode and connect it to the DC power supply, and use platinum wire as the cathode to connect it to the DC power supply. The distance between the two electrodes is 8cm, apply a voltage of 15V, and intercalate and expand at 10°C The electrode does not peel off for 1 hour, and graphene aggregates are obtained. After vacuum filtration and cleaning, disperse in a 100W ultrasonic cleaner for 1 hour to obtain a graphene dispersion. Graphene powder.
Embodiment 3
[0044] Sodium trifluoromethanesulfonate (NaCF 3 SO 3 ) as electrolyte, weigh 4gNaCF 3 SO 3 Add 1 g of water (the mass concentration of organic salt is 80%) and place the solution in an oven at 50° C. for 30 minutes until the solution is clear and transparent as a high-concentration brine electrolyte for electrochemical intercalation of graphite. Add the prepared electrolyte in the electrolytic cell, choose graphite paper as the anode and connect it to the DC power supply, and use silver wire as the cathode to connect it to the DC power supply. The distance between the two electrodes is 1cm, and a voltage of 5V is applied to expand the intercalation at 35°C. The electrode can last for 2 hours without peeling off, and graphene aggregates are obtained. After vacuum filtration and cleaning, they are dispersed in a 120W ultrasonic cleaner for 30 minutes to obtain a graphene dispersion. Graphene powder.
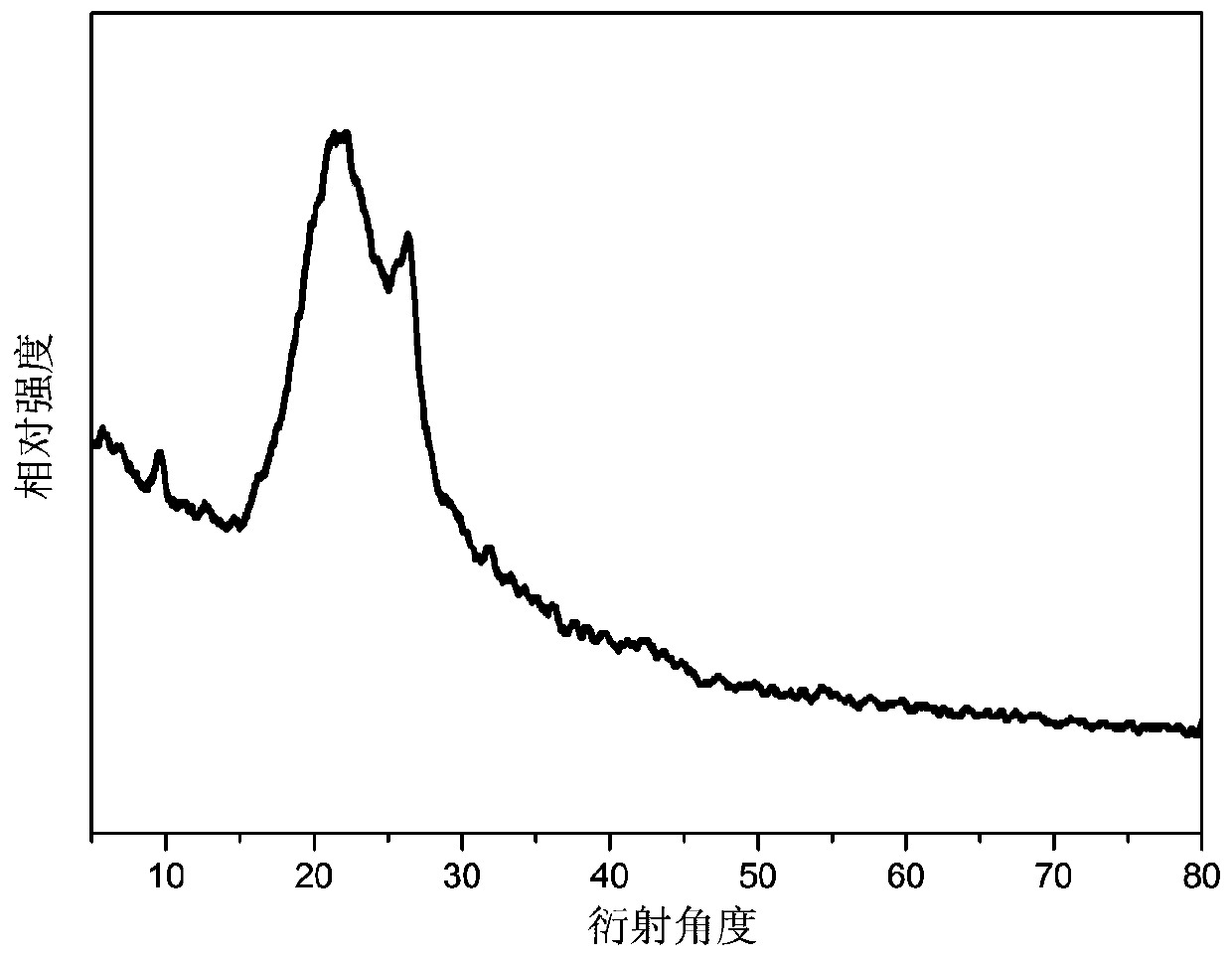
[0045] Scanning electron microscope analysis and X-ray diffraction analysi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com