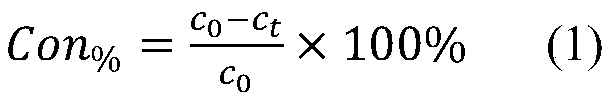Method for preparing 1,2-dichlorobenzene waste gas removal catalyst
A technology of catalyst and dichlorobenzene, which is applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve harmful problems and achieve the effect of simple operation process and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
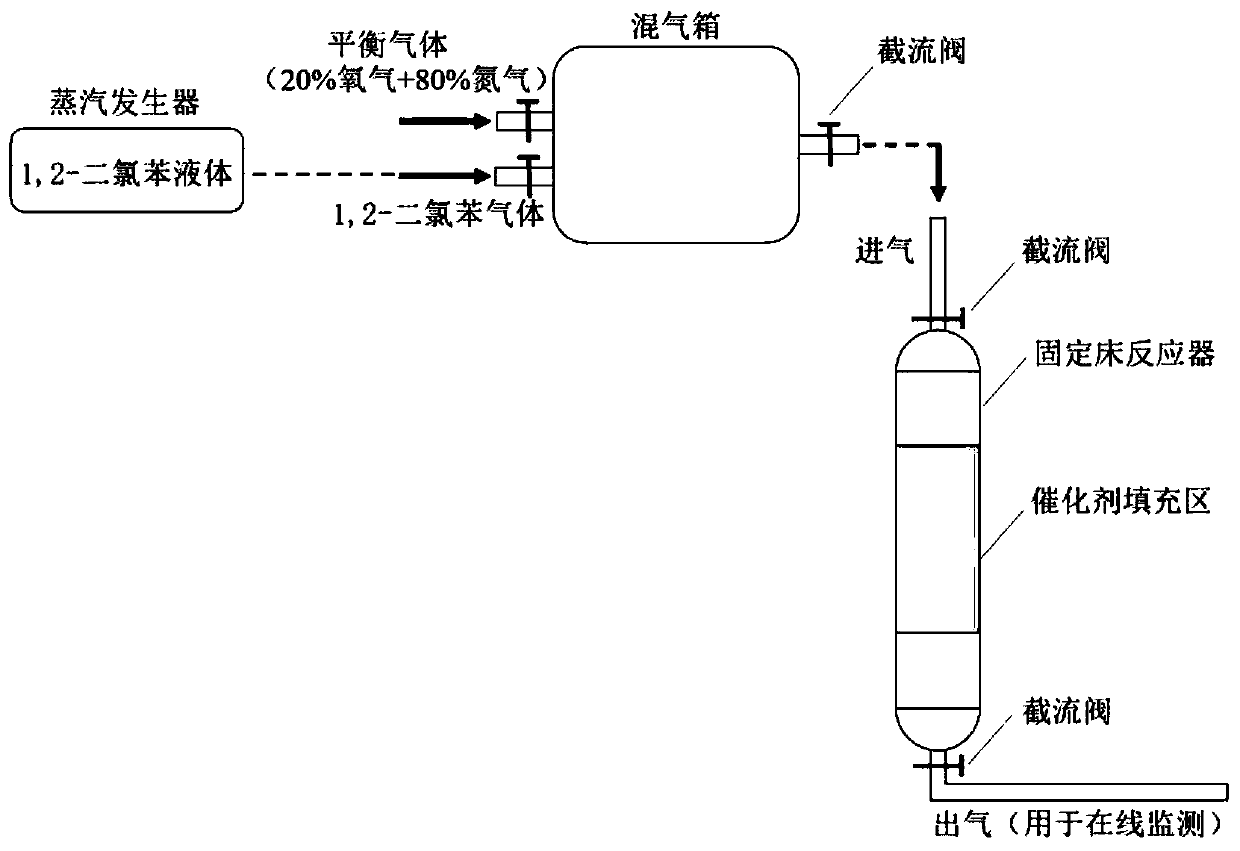
[0018] Effect of carbon dioxide gas exposure per unit volume on the removal rate of chromium in waste liquid and the performance of catalyst for the removal of 1,2-dichlorobenzene waste gas
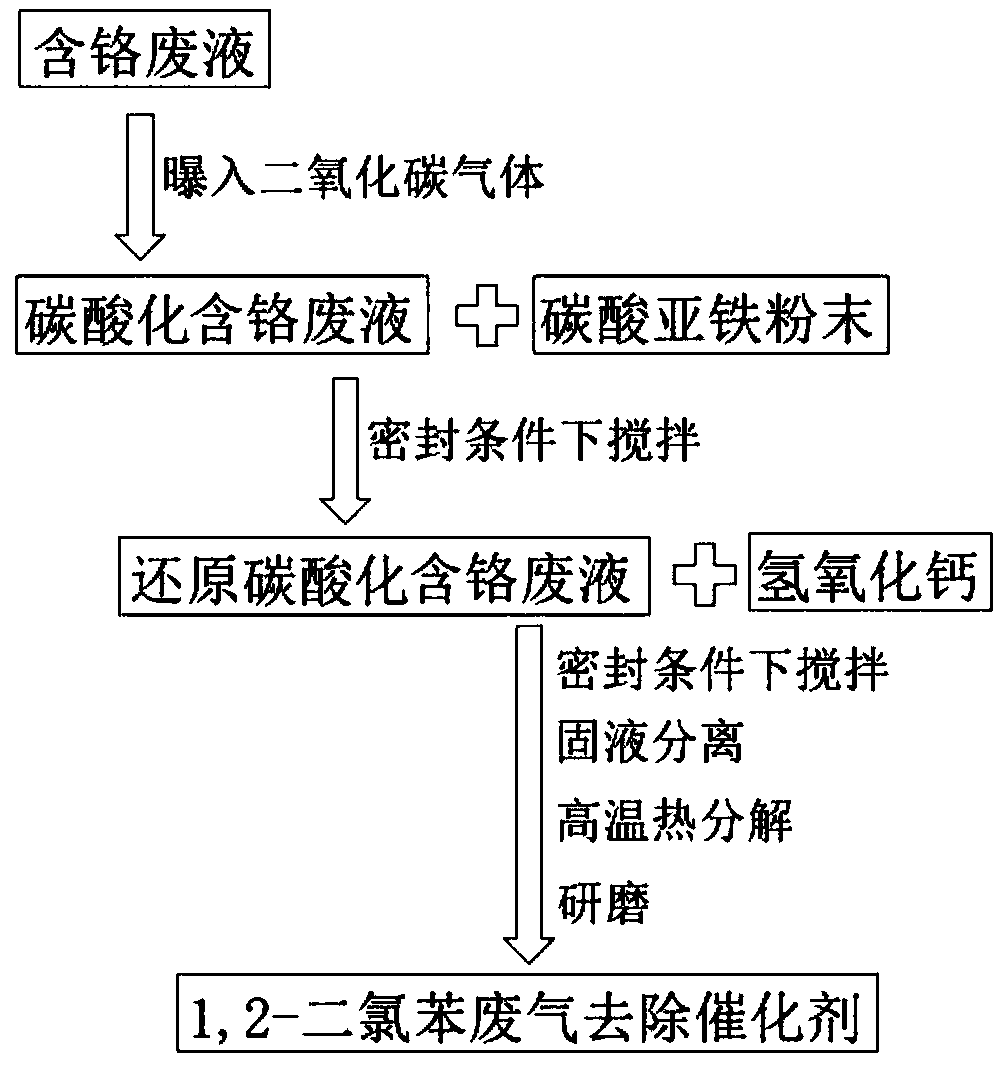
[0019] Preparation of waste liquid disposal and 1,2-dichlorobenzene waste gas removal catalyst: such as figure 1 As shown, take a unit volume of chromium-containing waste liquid and place it in a sealed container, and then expose 0.5, 0.7, 0.9, 1, 1.5, 2, 2.2, 2.5, 3 carbon dioxide gas per unit volume to obtain carbonation containing chromium waste liquid; according to ferrous ions and hexavalent chromium ion mol ratio 5:1 in the unit volume of chromium containing waste liquid, ferrous carbonate powder is placed in carbonation containing chromium waste liquid In the process, stir under sealed conditions until the ferrous carbonate powder is completely dissolved to obtain the reduced carbonation chromium-containing waste liquid; weigh the calcium hydroxide powder according to the molar rat...
Embodiment 2
[0028] Influence of the molar ratio of ferrous ions to hexavalent chromium ions in chromium-containing waste liquid on the removal rate of chromium in waste liquid and the performance of 1,2-dichlorobenzene waste gas removal catalyst
[0029] Waste liquid disposal and preparation of 1,2-dichlorobenzene waste gas removal catalyst: take a unit volume of chromium-containing waste liquid and place it in a sealed container, then expose 2 unit volumes of carbon dioxide gas into the container to obtain carbonation containing Chromium waste liquid, according to the molar ratio of ferrous ions to hexavalent chromium ions in unit volume of chromium-containing waste liquid: 4:1, 4.5:1, 4.8:1, 5:1, 6:1, 7:1, 7.2:1, 7.5:1, 8:1 Weigh ferrous carbonate powder respectively and place it in the carbonation chromium-containing waste liquid, stir under sealed conditions until the ferrous carbonate powder is completely dissolved, and obtain the reduced carbonation chromium-containing waste liquid. ...
Embodiment 3
[0035] Effect of molar ratio of ferrous ion to calcium ion on the removal rate of chromium in waste liquid and the performance of catalyst for the removal of 1,2-dichlorobenzene exhaust gas
[0036] Waste liquid disposal and preparation of 1,2-dichlorobenzene waste gas removal catalyst: take a unit volume of chromium-containing waste liquid and place it in a sealed container, then expose 2 unit volumes of carbon dioxide gas into the container to obtain carbonation containing Chromium waste liquid, according to the molar ratio of ferrous ions and hexavalent chromium ions in unit volume of chromium-containing waste liquid is 7:1, weigh ferrous carbonate powder and place it in carbonated chromium-containing waste liquid, and stir under sealed conditions until ferrous carbonate powder Completely dissolve to obtain the reduced carbonated chromium-containing waste liquid, according to the molar ratio of ferrous ion to calcium ion 1:2, 1:2.5, 1:2.8, 1:3, 1:4, 1:5, 1:5.2, 1 :5.5, 1:6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com