A kind of pentapeptide analogue of insect antipharyngeal side voxel and its application
A carrier and drug technology, applied in applications, peptides, pesticides, etc., can solve the problems of complex structure, unsuitability for development, high synthesis cost, and achieve the effect of simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
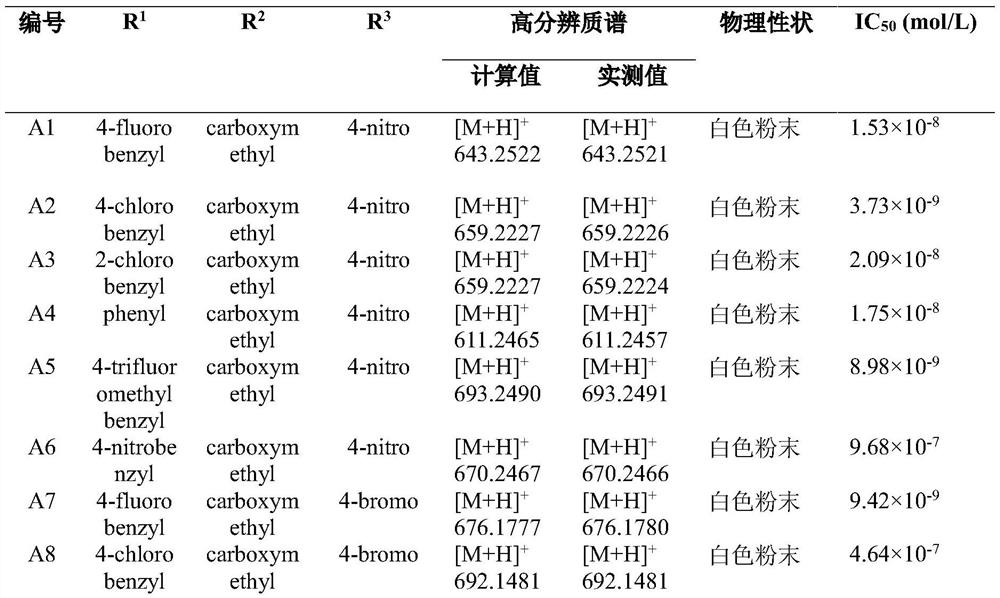
[0043] The compound of formula A of the present invention is prepared according to the method of polypeptide solid-phase synthesis.
[0044] Taking compound A1 as an example, its preparation method is as follows:
[0045] (1) Resin activation: Weigh 440mg Rink Amide-AM resin, activate it with 5mL DCM for 3h, add 20% piperidine DMF solution to cut for 20min, wash with DMF and DCM for 3 times, monitor the reaction with Kaiser’s reagent.
[0046] (2) Connect to Leu: add Fmoc-Leu-OH (3 times equivalent), HBTU (3 times equivalent), HOBt (3 times equivalent), DIEA (6 times equivalent), dissolve in 5mL DMF, stir at room temperature for 2h, remove The reaction solution was washed three times with DMF and DCM, and the reaction was monitored by Kaiser's reagent. Then add 20% piperidine DMF solution to cut for 20min, and wash 3 times with DMF.
[0047] (3) Gly connection: add Fmoc-Gly-OH (3 times equivalent), HBTU (3 times equivalent), HOBt (3 times equivalent), DIEA (6 times equivalen...
Embodiment 2
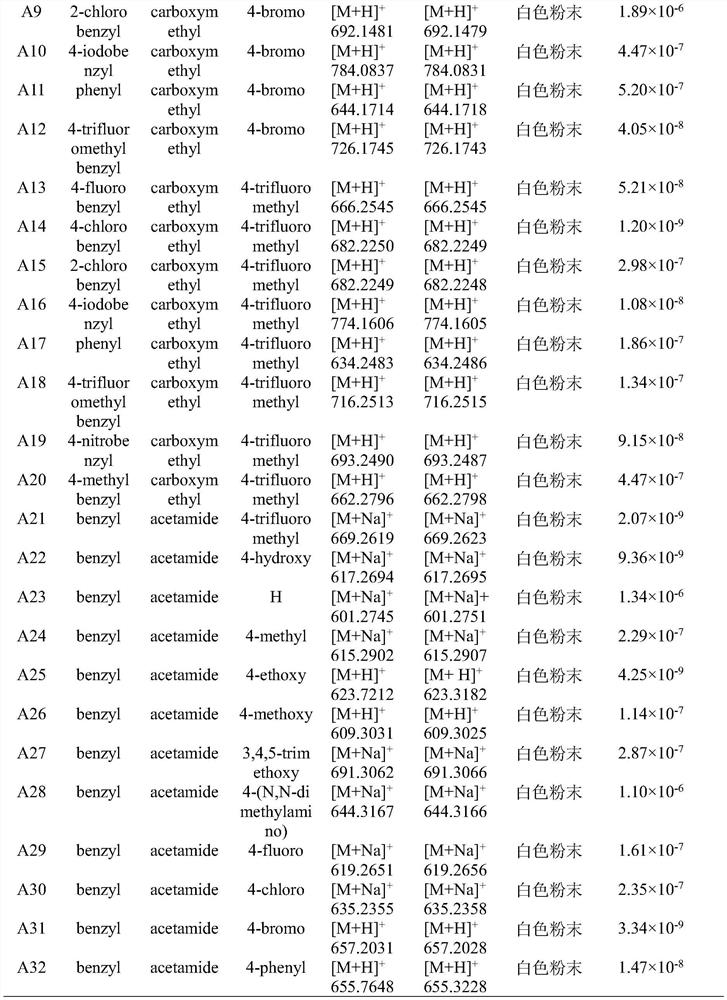
[0058] Example 2 In vitro inhibitory activity of the compounds of the present invention on the biosynthesis of juvenile hormone in the Pacific cockroach
[0059] The present invention adopts the GC-MS / MS bioassay method of Kai Zhenpeng et al. (reference: Kai, Z.P.; Yin, Y.; Zhang, Z.R.; Huang, J.; Tobe, S.S.; Chen, S.S.J. , 1538, 67). The newly emerged female adults were collected, and after the 7th day, they were dissected to obtain the pharyngeal body. The target compound was made into a 10mmol / L mother solution, which was successively diluted into 1mmol / L, 0.1mmol / L, 0.01mmol / L, 0.001mmol / L and 0.0001mmol / L assay solutions. Then use a 1-10uL pipette gun to take 10uL of different concentrations of the measurement solution and add it to a 1.5mL centrifuge tube containing 990uL M199 medium. After mixing thoroughly, the obtained concentrations are 0.01mmol / L, 0.001 mmol / L, 0.0001 mmol / L, 0.00001 mmol / L, and 0.000001 mmol / L. Measure 100uL of the prepared medicinal solution an...
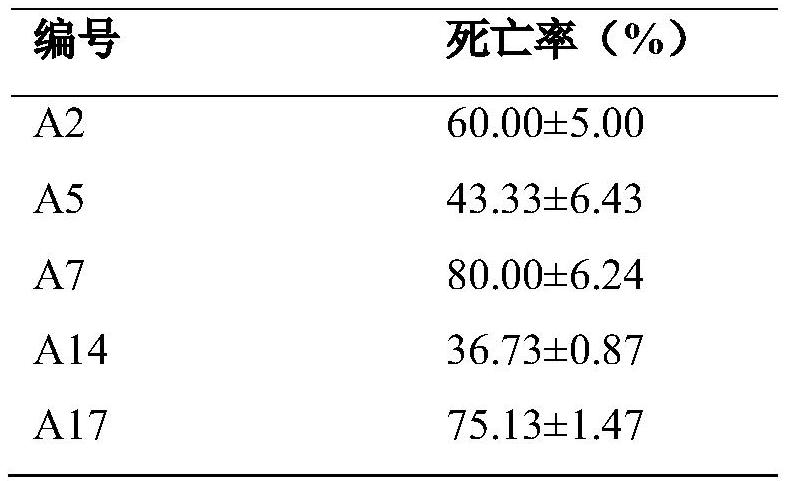
Embodiment 3
[0061] Embodiment 3 The biological activity of some compounds of the present invention to Plutella xylostella under the condition of 200mg / L
[0062] The application of the pentapeptide analogue of a class of insect depressor pharyngeal voxin as described in any one of claims 1-3 in the prevention and treatment of diamondback moth. The screening method for insecticidal activity against Plutella xylostella is as follows: use the leaf soaking method to conduct preliminary screening of the activity of samples at a concentration of 200mg / L; select standard test insects that are continuously reared in the room and have consistent physiological states. The original drug is dissolved with a small amount of DMF, and then diluted with 0.05% TritonX-100 aqueous solution. Each concentration was repeated 4 times, with 30 test insects for each repetition, and a blank control was set; the cabbage leaves that had grown straight were selected, immersed in 200mg / L liquid medicine for 5s, dried...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com