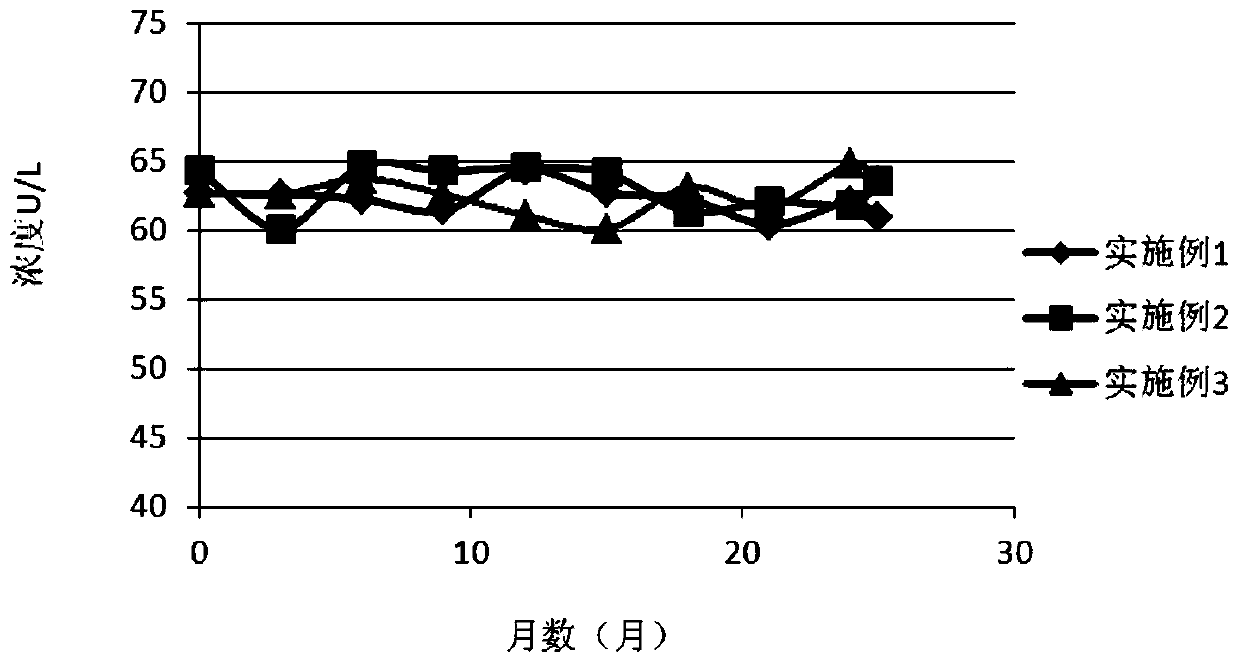
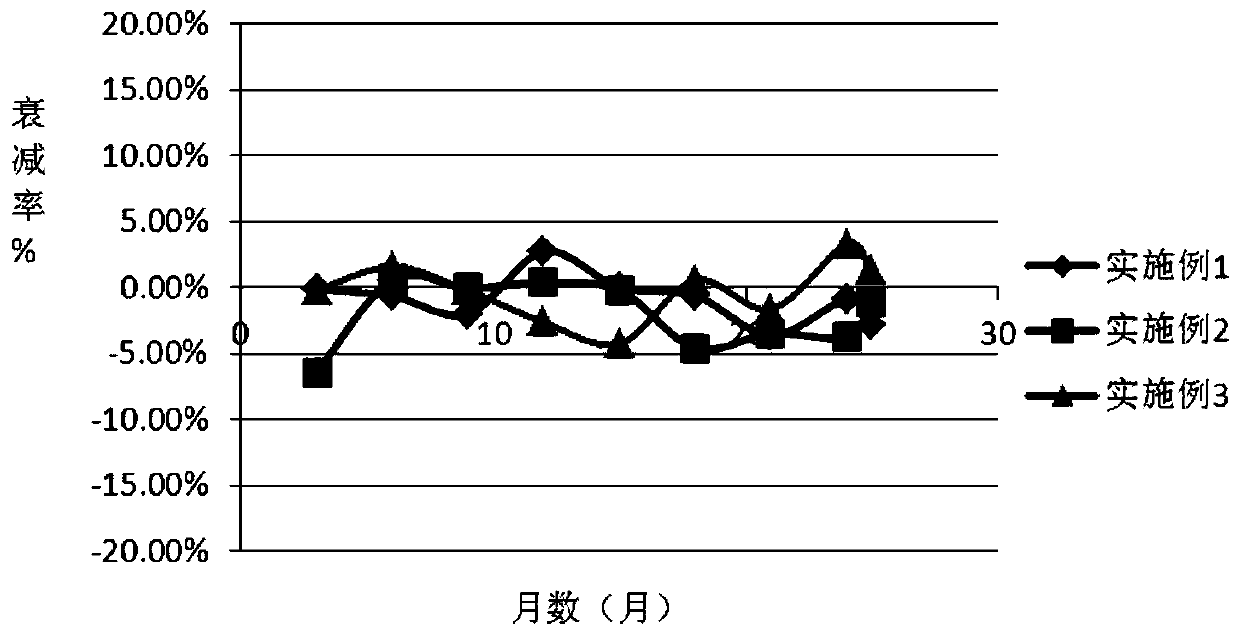
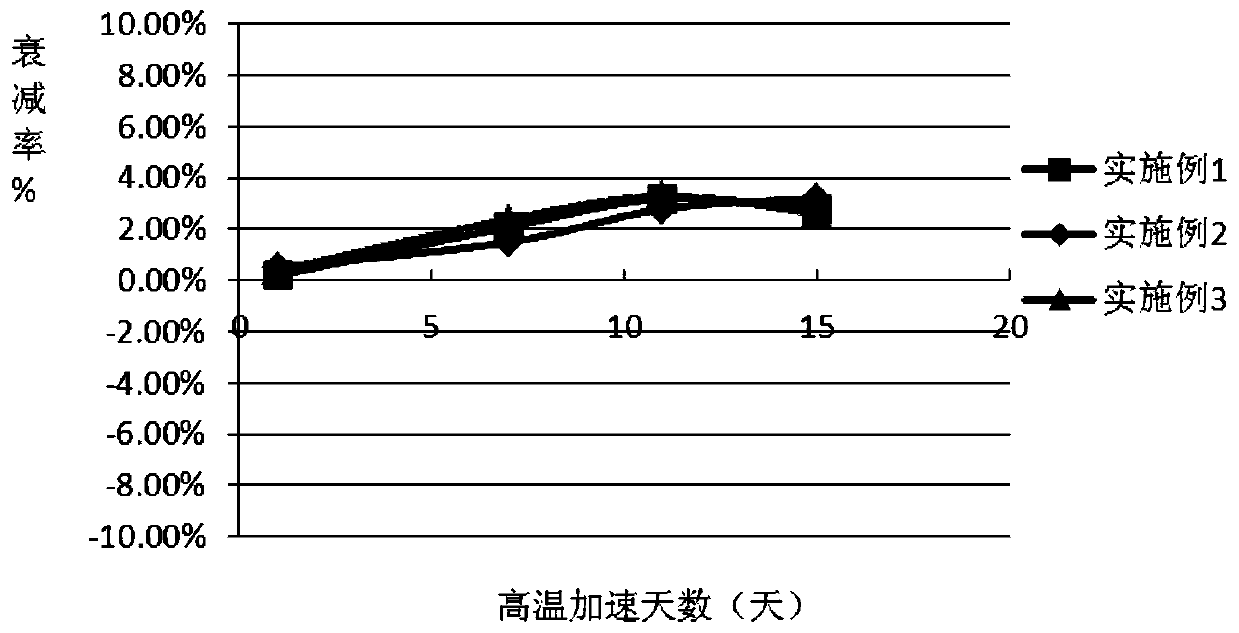
5'-nucleotidase quality control product
A technology of nucleotidase and quality control products, applied in the field of 5'-nucleotidase quality control products, can solve the problems of short validity period and achieve the effects of improving stability, avoiding damage, and protecting stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The preparation of the 5'-nucleotidase quality control product of the present invention can be prepared according to the conventional process method in this field, without special restrictions, preferably:
[0036] Add matrix serum into a clean preparation container, then add buffer, preservative, protective agent 1, protective agent 2, and surfactant in sequence, and stir until completely dissolved; use a 0.22um filter membrane to filter once, and use Dilute HCL solution or 0.1% NaOH solution to adjust the pH value to 7.5-7.6 (20±0.5°C), add 5'-nucleotidase according to the concentration calculation, so that the concentration of the quality control product is 45-72U / L, according to 1ml / bottle specifications for packaging.
Embodiment 1
[0039] A long-acting and stable 5'-nucleotidase quality control product, the composition of which is as follows:
[0040]
[0041] The preparation process of this quality control product is:
[0042] Add the whole batch of matrix serum into a clean preparation container, then add the whole batch of buffer solution, preservative, protective agent 1, protective agent 2, and surfactant in sequence, and stir until completely dissolved; use 0.22um filter membrane once. Use dilute HCL solution or 0.1% NaOH solution to adjust the pH value to 7.5-7.6 (20±0.5°C), and add 5’-nucleotidase according to the concentration calculation, so that the concentration of the quality control product is 45-72U / L. Pack according to the specification of 1ml / bottle.
Embodiment 2
[0044] A long-acting and stable 5'-nucleotidase quality control product, the composition of which is as follows:
[0045]
[0046]
[0047] The preparation process of this quality control product is:
[0048] Add the whole batch of matrix serum into a clean preparation container, then add the whole batch of buffer solution, preservative, protective agent 1, protective agent 2, and surfactant in sequence, and stir until completely dissolved; use 0.22um filter membrane once. Use dilute HCL solution or 0.1% NaOH solution to adjust the pH value to 7.5-7.6 (20±0.5°C), and add 5’-nucleotidase according to the concentration calculation, so that the concentration of the quality control product is 45-72U / L. Pack according to the specification of 1ml / bottle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com