Recombinant human truncated-type keratinocyte growth factor-1 eye drops and preparing method and application thereof
A keratinocyte and growth factor technology, applied in drug combination, pharmaceutical formulation, peptide/protein composition, etc., can solve the problem of not promoting blood vessel growth, improve stability and biological activity, promote corneal repair, and have good industrial application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
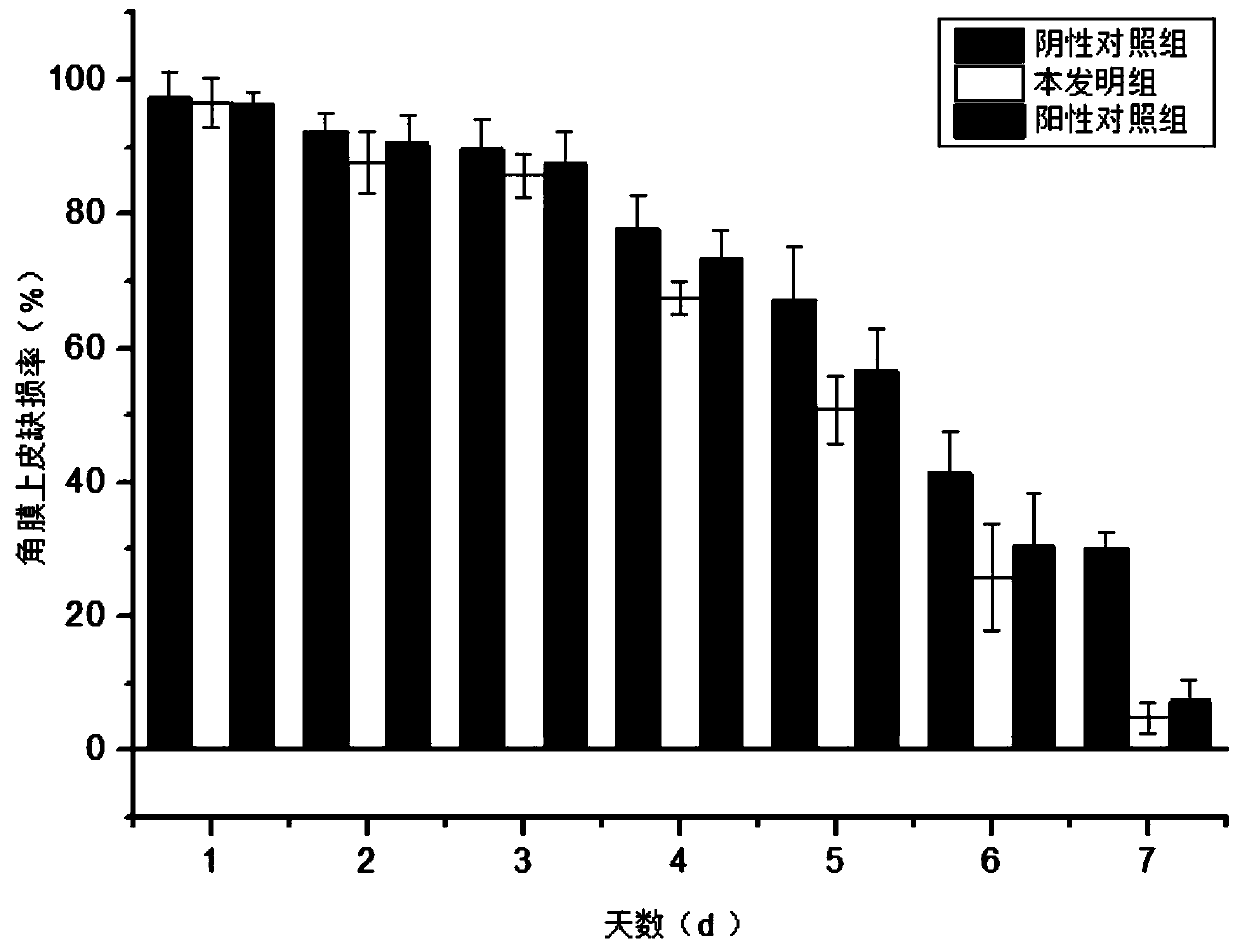
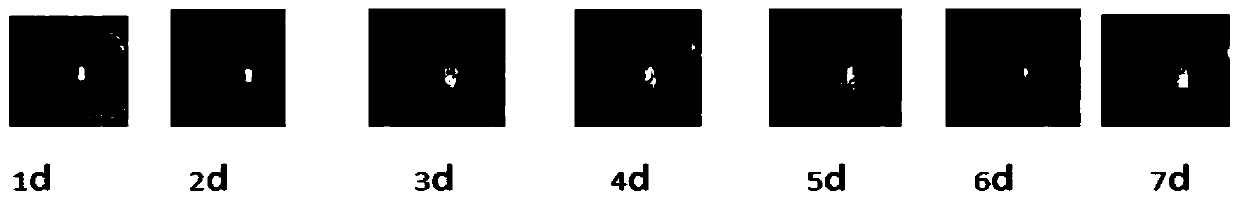
Image
Examples
Embodiment 1
[0054] Example 1: A preparation method of recombinant human truncated keratinocyte growth factor-1 eye drops:
[0055] S1 Disperse polyvinyl alcohol in 500mL of water for injection, autoclave at 121°C, and cool to room temperature to obtain thickener stock solution;
[0056] S2 Dissolve recombinant human truncated keratinocyte growth factor-1, citrate buffer system, histidine, mannitol, sodium chloride, Tween 20, and 2 mg sucrose in 400 mL of water for injection, and use a 0.22 μm filter membrane to dissolve Bacterial filtration to obtain recombinant human truncated keratinocyte growth factor-1 mixed solution;
[0057] S3 Under aseptic conditions, mix the thickener stock solution obtained in S1 and the recombinant human truncated keratinocyte growth factor-1 mixture in S2 evenly, adjust to 1 L with sterile water for injection, and then use 0.22 μm sterile grade filter to obtain recombinant human truncated keratinocyte growth factor-1 eye drops;
[0058] S4 Aseptically fillin...
Embodiment 2
[0060] Embodiment 2: The difference with Embodiment 1 is that each component and its consumption are as shown in Table 1.
Embodiment 3
[0061] Embodiment 3: The difference from Embodiment 1 is that each component and its consumption are as shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com