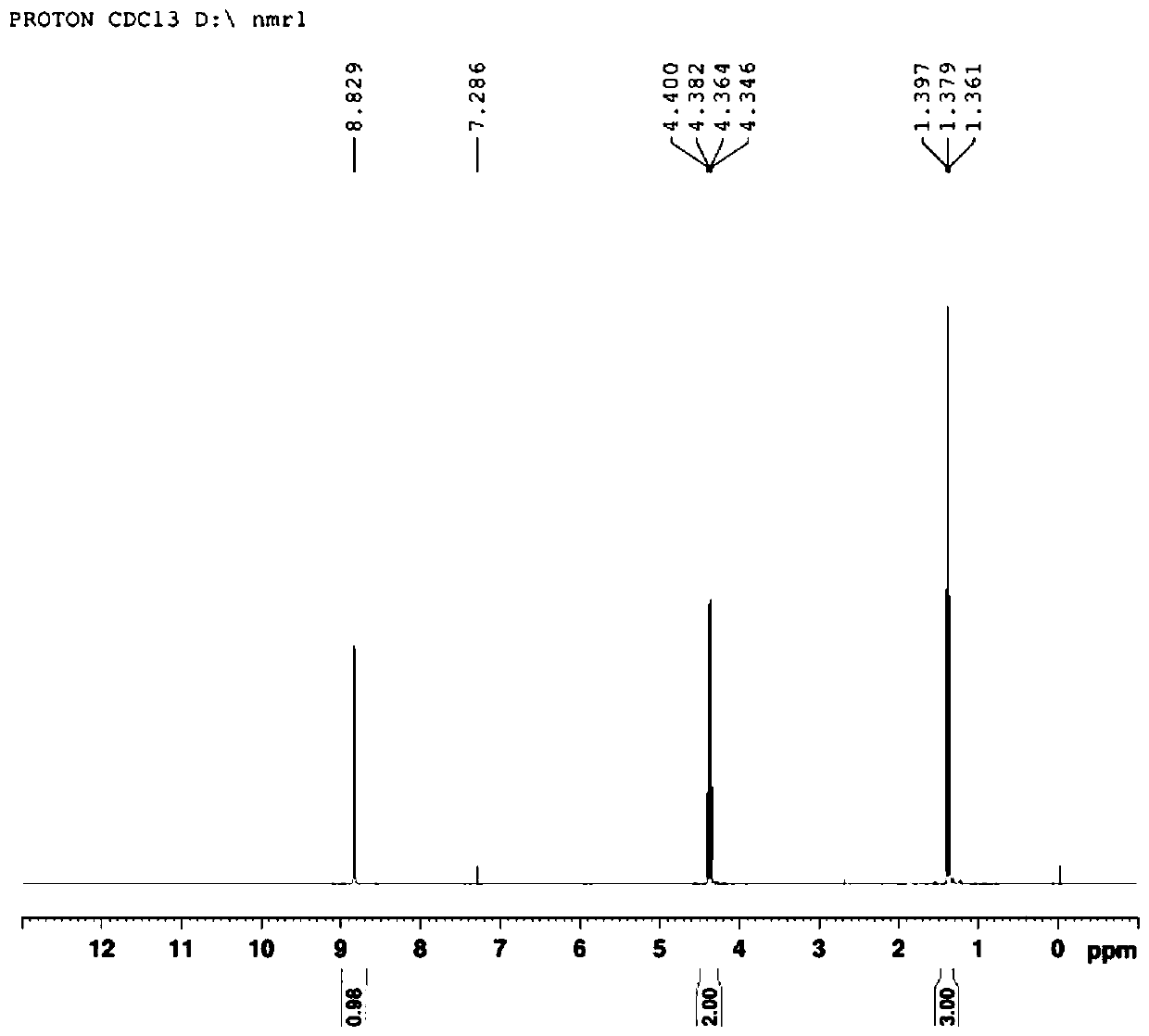
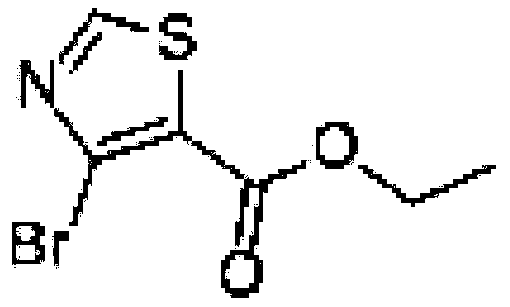
Synthetic method for 4-bromo-5-thiazolecarboxylic acid ethyl ester
A technology of ethyl thiazole carboxylate and a synthesis method, which is applied in the field of pharmaceutical intermediates, can solve the problems of not finding synthesis route data and the like, and achieves the effects of low cost of raw materials and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] A kind of synthetic method of 4-bromo-5-thiazole carboxylic acid ethyl ester, comprises the steps:
[0017] ①Mix cuprous bromide, acetonitrile and isoamyl nitrite, heat to 60°C, add ethyl 4-aminothiazole-5-carboxylate in batches, and react for 30 minutes after the addition, and cool the reaction solution after the reaction of the raw materials is complete to room temperature.
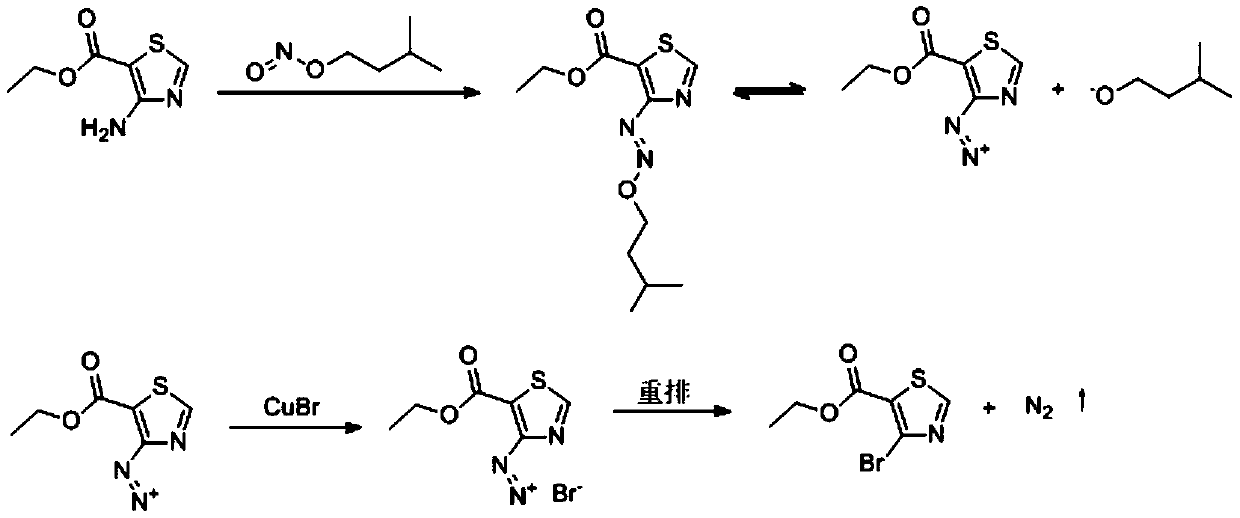
[0018] In step ①, two-step reactions mainly take place, and the chemical reaction formula is expressed as:
[0019]
[0020] The reaction will eventually produce nitrogen gas that does not need to be handled.
[0021] 2. the reaction solution obtained by step 1. is added in hydrobromic acid, extracted with ethyl acetate, and gets the organic phase;
[0022] ③ The organic phase obtained in step ② was washed with water, dried over anhydrous sodium sulfate, and concentrated through a column to obtain ethyl 4-bromo-5-thiazolecarboxylate.
Embodiment
[0025] ①Add 10.6g of cuprous bromide into a 250ml three-necked flask, add 100ml of acetonitrile and 6.9g of isoamyl nitrite, heat to 60°C, add 28g of ethyl 4-aminothiazole-5-carboxylate in batches, and complete the reaction 30min, wait for the raw materials to react completely, and cool the reaction solution to room temperature;
[0026] ②Add 80ml of 20wt% hydrobromic acid to the reaction solution, extract 3 times with 300ml of ethyl acetate, and take the organic phase;
[0027] ③ Wash the organic phase with water, dry it with anhydrous sodium sulfate, and concentrate to obtain the crude product. Use 200-300 mesh silica gel as the stationary phase, petroleum ether / ethyl acetate (volume ratio 20:1, 15:1, 10:1, 5:1) was used as the eluent to wash the column successively to obtain 36 g of ethyl 4-bromo-5-thiazolecarboxylate, with a yield of 95%.
[0028] In step ①, ethyl 4-aminothiazole-5-carboxylate is added in batches to the reaction solution, and the latter batch is added aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com