XPO1 gene knockout pancreatic cancer cell line and construction method thereof
A technology of pancreatic cancer cells and construction methods, applied in the direction of microbial-based methods, tumor/cancer cells, and other methods of inserting foreign genetic materials, which can solve the problems of high off-target rate, unknown curative effect, and unstable gene knockout efficiency and other issues to achieve the effect of improving regulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
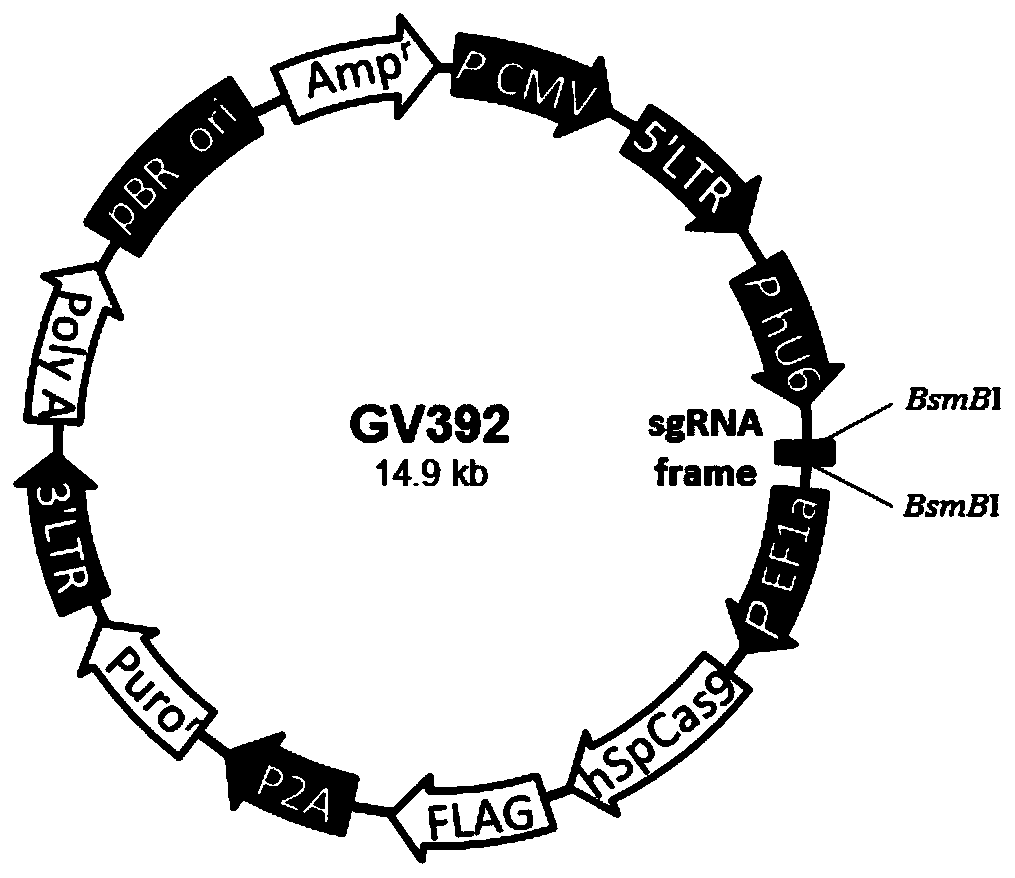
[0033] Description of the experimental process: Aiming at the target gene sequence of the target gene, the sgRNA interference target sequence is designed, and a single-stranded DNA oligo is synthesized by a special primer synthesis company. Enter the Lenti-CAS9-sgRNA-tag vector (element sequence: U6-sgRNA-EF1a-Cas9-FLAG-P2A-puro). The ligated product was competently transformed with TOP10, and the colony PCR was positively cloned and then sequenced to obtain an overexpression lentiviral plasmid expressing sgRNA with the correct sequence.
[0034] The sgRNA sequence is as follows: sgRNA: GGATTATGTGAACAGAAAAG (SEQ ID NO.1).
[0035] Synthetic oligo information is shown in Table 1 below:
[0036] Table 1
[0037] No. 5’ STEM 3’ XPO1-sgRNA-a CACCg GGATTATGTGAACAGAAAAG XPO1-sgRNA-b aaac CTTTTCTGTTCACATAATCC c
[0038] The experimental steps are as follows:
[0039] 1. sgRNA design and synthesis
[0040] Find the sequence of the human XPO1...
Embodiment 2
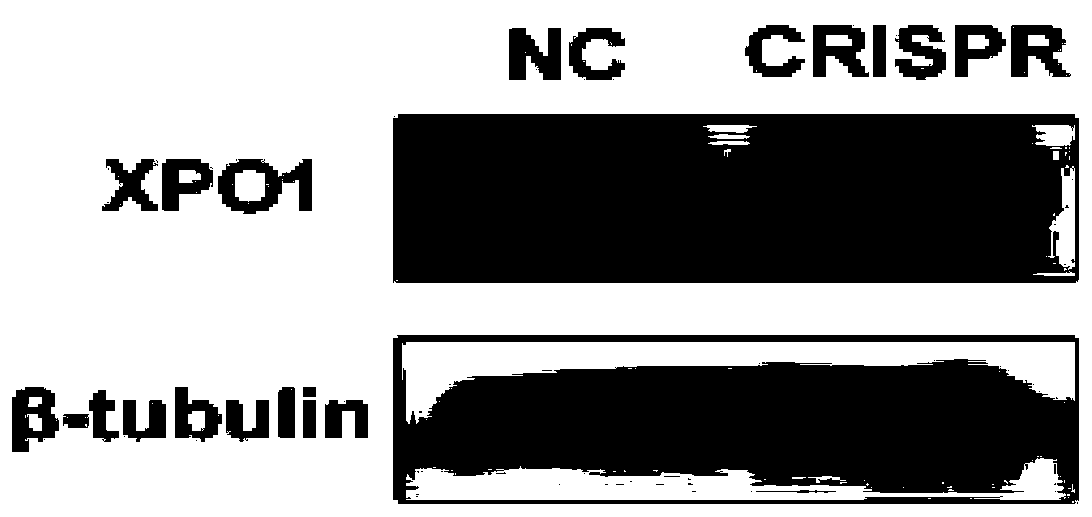
[0072] Example 2 Western blots detection of XPO1 knockout cells obtained in Example 1
[0073] The experimental steps are as follows:
[0074] 1. Protein heating and denaturation treatment: prepare a 1.5ml clean EP tube, draw an equal mass of protein (100ug) according to the protein concentration, and the volume of each tube is 100ul, fill up the final volume of each group of proteins with RIPA solution, add 1 / 5 Mix the final volume of 5×SDS-PAGE loading buffer evenly, denature the protein in a metal bath at 98°C for 10 minutes, take out the protein and put it on ice, centrifuge briefly, cool to room temperature, load directly or store at -20 ℃.
[0075] 2. Gel preparation: prepare 10% separating gel and 5% stacking gel, see materials section for configuration method. Put it in the waste electrophoresis solution (see the material section for the preparation method) overnight at 4°C.
[0076] 3. Sample loading: 20ul protein solution per well, add 5ul and 3ul pre-stained mark...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com