A kind of flavonoid monoterpene compound and its preparation method and application
A technology of compound and monoterpene, applied in the field of natural medicinal chemistry, can solve the problem that the antibacterial mechanism has not been studied, and achieve the effect of strong DPPH free radical scavenging ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
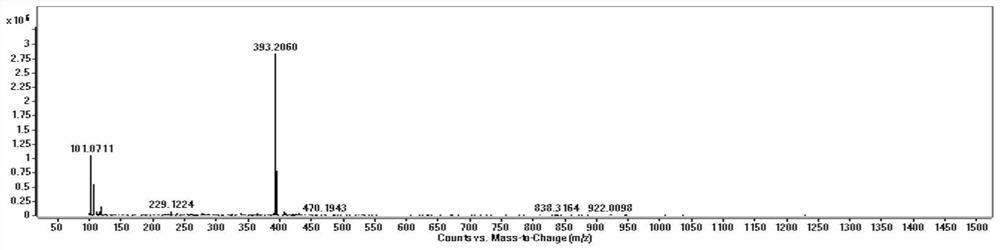
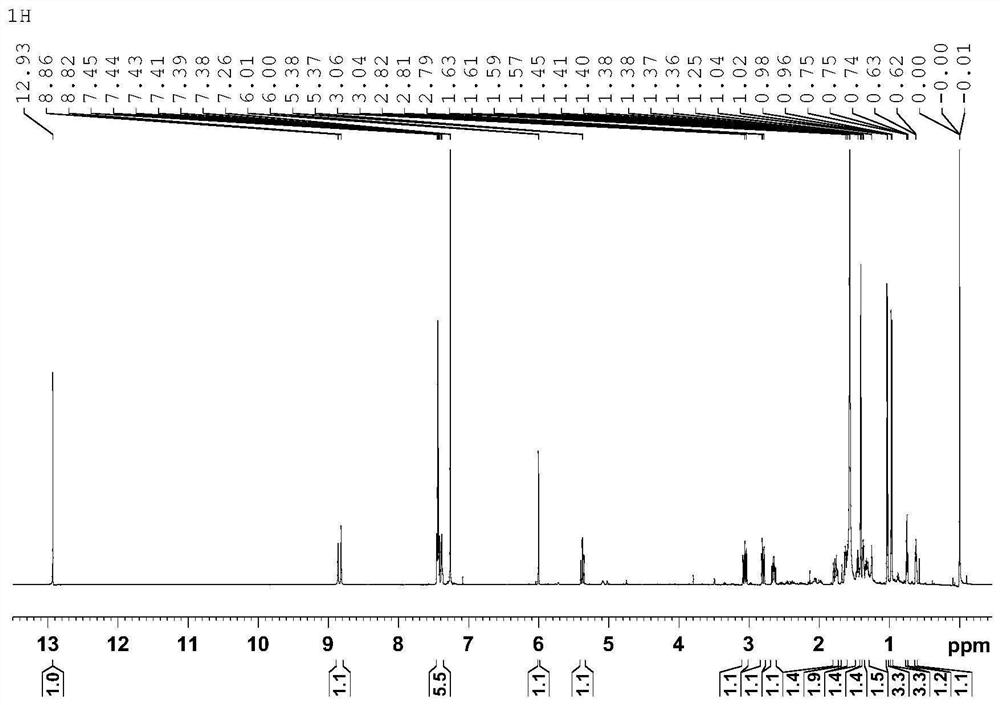
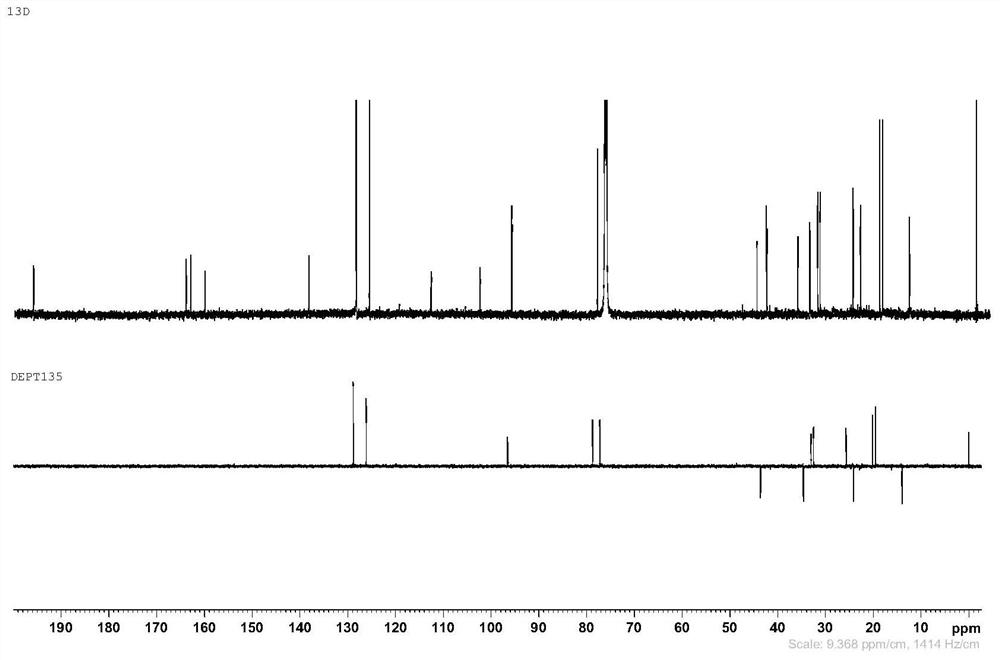
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 grammatin A
[0031] 1. Heat and reflux the dried whole grass powder of C. gramatus with 80% ethanol solution to extract 3 times, each time for 2 hours, filter and combine the extracts, recover ethanol under reduced pressure, then add water to disperse and then use etc. Petroleum ether, ethyl acetate and n-butanol were extracted to obtain petroleum ether phase, ethyl acetate phase and n-butanol phase, respectively.
[0032] 2. The petroleum ether phase extract (35.5g) obtained in 1. was subjected to silica gel column chromatography, and then eluted by petroleum ether-acetone gradient, the volume ratio was changed from 100:1 to 0:1, and thin-layer chromatography was inspected. According to the order of elution, they were combined into 5 components, and the second component was subjected to reverse-phase C8 silica gel column chromatography, and then eluted by methanol-water gradient system, and the volume ratio was changed from 30:70 to 100...
Embodiment 2
[0044] The mensuration of embodiment 2 DPPH free radical scavenging ability
[0045] Accurately weigh 4mg of DPPH and dissolve it in methanol to a 100mL volumetric flask, which is the DPPH solution.
[0046] Take compound (grammatin A, 2mg / mL) 0.1, 0.2, 0.3, 0.4, 0.5mL (make up the insufficient volume to 0.5mL with methanol) and 2mL DPPH solution in the same test tube, shake well. Place it in the dark for 30min, and measure the absorbance at 517nm with the corresponding blank solution as a control. The formula for calculating the DPPH free radical inhibition rate is as follows:
[0047] Inhibition rate% = [A 0 -(A b -A * )] / A 0 *100%
[0048] Among them: A 0 is the absorbance after reaction without sample inhibitor;
[0049] Ab is the absorbance after adding the sample inhibitor to the reaction;
[0050] A * is the absorbance of no inhibitor and DPPH.
[0051] After the above experiments, it was found that the compound has a strong ability to scavenge DPPH free radi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com