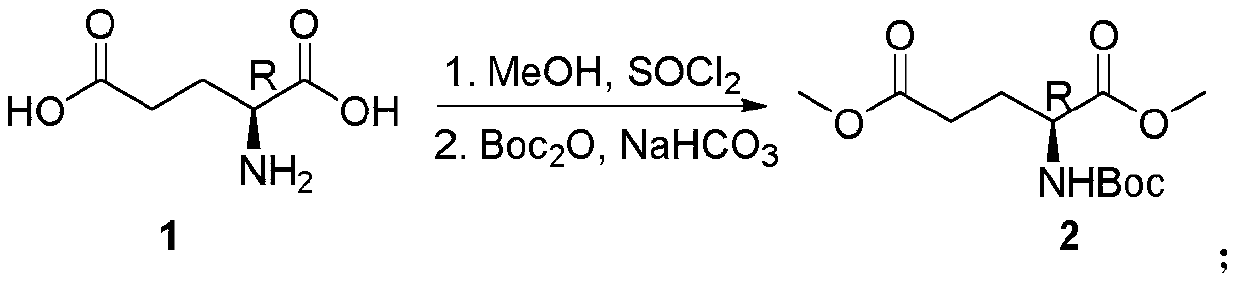Synthetic process of anti-hyperglycemic drug intermediate R-3-amino-piperidine dihydrochloride
An anti-hyperglycemia, R-3- technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of low total yield and limited application, and achieve high total yield, cheap raw materials, and atom economy Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0051] The present invention will be further described below in conjunction with specific embodiments. The following examples are only used to illustrate the technical solution of the present invention more clearly, but not to limit the protection scope of the present invention.
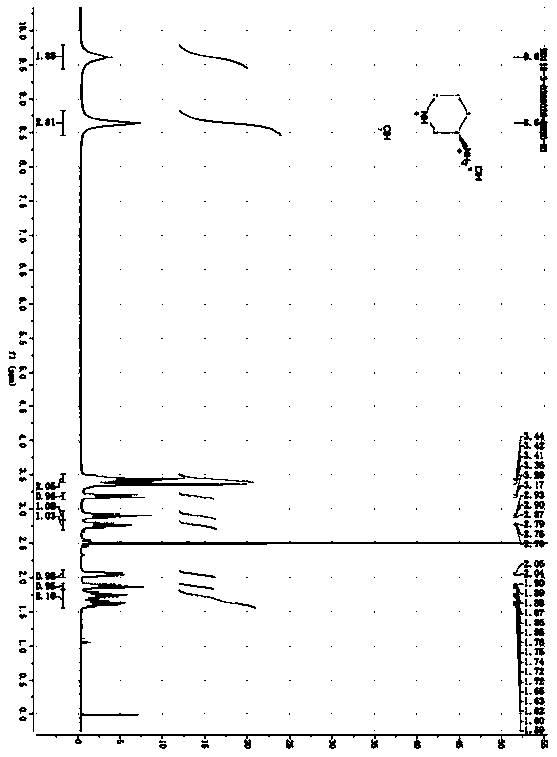
[0052] 1, the preparation of dimethyl (tert-butoxycarbonyl)-L glutamate:
[0053] Add 147g (1mol) of L-glutamic acid and 588g of methanol into a three-necked flask; stir at room temperature, and slowly add 240g (2.02mol) of thionyl chloride into the reaction system through the dropping funnel; The internal temperature was raised to 55° C., and the reaction was kept at 55° C. for 2 hours; the end of the reaction was monitored by thin layer chromatography (TLC). After the reaction was completed, the solvent was evaporated under reduced pressure, and the obtained crude product was dissolved in water (825 g). Take 165g of solid sodium bicarbonate and add it into water to adjust the pH to 7-8, then add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com