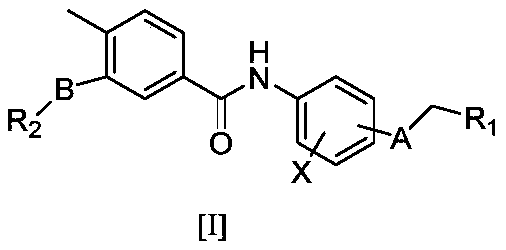
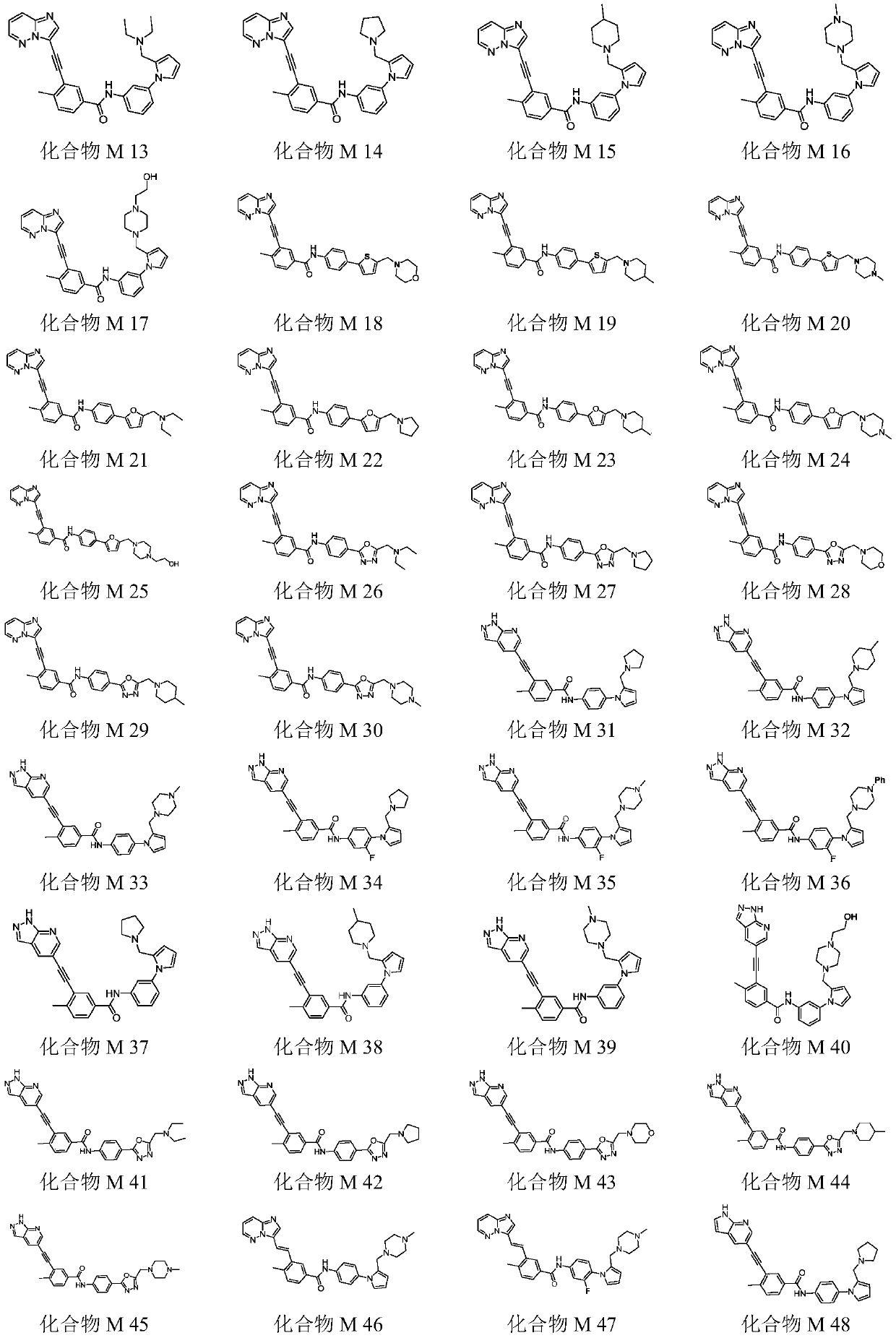
Novel substituted benzamide compounds and pharmaceutically-acceptable salts, and preparation method and application thereof
A benzamide and compound technology, applied in the field of new substituted benzamide compounds, can solve the problems of steric hindrance, death, adverse reactions of patients, etc., and achieve a wide therapeutic window, wide anti-cancer spectrum, excellent anti-tumor activity and safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
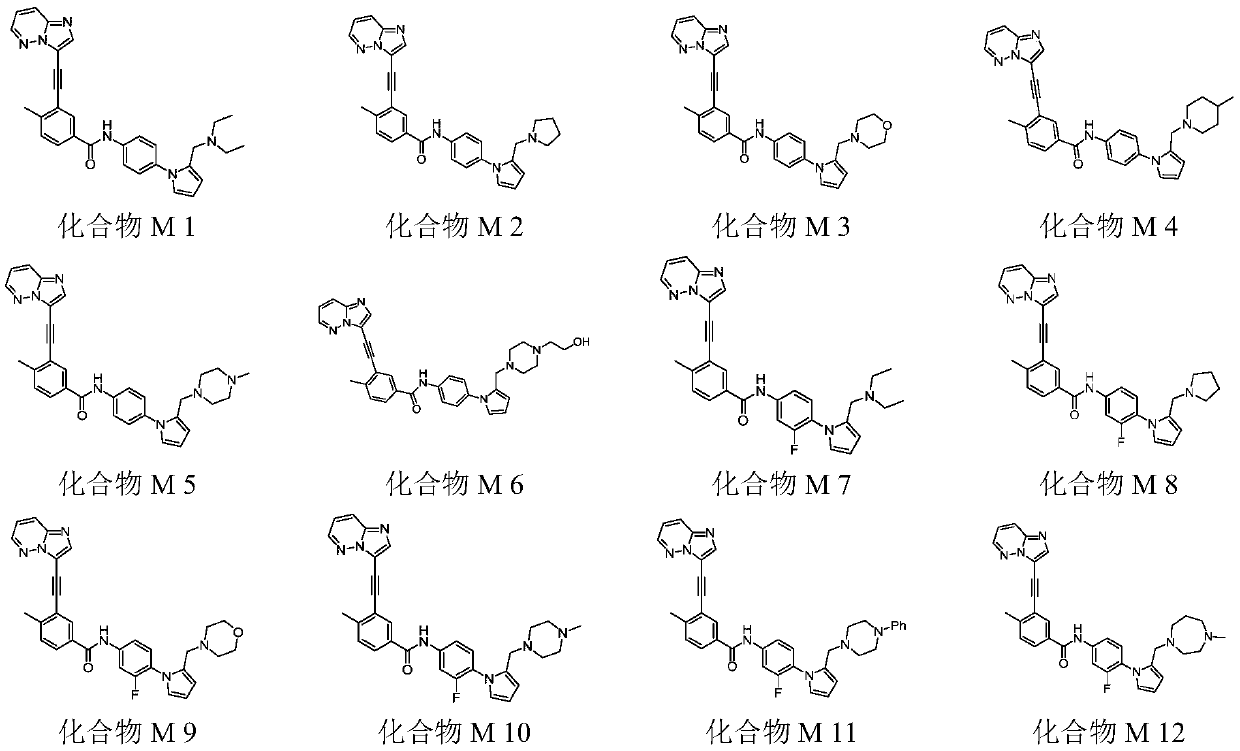
[0048] Example 1 Synthesis of compound M1
[0049]
[0050] 1. Synthesis of intermediate a1
[0051] The 3-bromoimidazo[1,2-b]pyridazine (36.64g, 0.186mol), Pd(pph 3 ) 4 (10.73g, 9.29mmol), CuI (5.30g, 0.028mmol) and DIPEA (32.4mL, 0.279mol) were added to N,N dimethylformamide (150mL), under nitrogen protection, added trimethylsilylacetylene ( 21.89g, 0.223mol), react at room temperature for 1h, pour the reaction solution into 200mL water, extract with ethyl acetate (100mL×3) to separate the organic phase, dry with anhydrous sodium sulfate, filter, concentrate, and separate the product by column chromatography on silica gel 28.22g, yield 71%, MS (ESI) m / z (%): 216.3[M+H] + .
[0052] 2. Synthesis of intermediate b1
[0053] The intermediate a1 (28.38g, 0.132mol) and K 2 CO 3 (36.43g, 0.264mol) was added to MeOH (150mL), reacted at room temperature for 0.5h, the reaction solution was concentrated, the crude product was separated by column chromatography on silica gel to obtain 16.88g,...
Embodiment 2
[0066] Example 2 Synthesis of Compound M2
[0067]
[0068] 1. Synthesis of intermediate g2
[0069] Add formaldehyde (0.07g, 2.25mmol), R 1 (Tetrahydropyrrole) (0.16g, 2.25mmol) was added to glacial acetic acid (10mL), reacted at room temperature for 0.5h, intermediate f 1 (0.6g, 1.5mmol) was added, reacted at 35℃ for 4h, and glacial acetic acid was evaporated under reduced pressure , Add saturated Na 2 CO 3 The solution adjusted the pH of the reaction night to 8-9, extracted with dichloromethane (50mL×3), separated the organic phase, dried over anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography on silica gel to obtain a white solid 0.44g with a yield of 61.7% ,MS(ESI)m / z(%):486.2[M+H] + .
[0070] 2. Synthesis of compound M2
[0071] Intermediate g2 (0.24g, 0.5mmol), intermediate b1 (0.093g, 0.65mmol), Pd(ph 3 ) 4 (0.029g, 0.025mmol), CuI (0.007g, 0.038mmol), DIPEA (0.19g, 1.5mmol), DMF (15mL), under nitrogen protection, react at 55℃ for 4.5h, ...
Embodiment 3
[0072] Example 3 Synthesis of Compound M3
[0073]
[0074] 1. Synthesis of intermediate g3
[0075] Add formaldehyde (0.07g, 2.25mmol), R 1 (Morpholine) (0.20g, 2.25mmol) was added to glacial acetic acid (10mL) and reacted at room temperature for 0.5h. Intermediate f1 (0.6g, 1.5mmol) was added and reacted at 35℃ for 4h. The glacial acetic acid was evaporated under reduced pressure and saturated Na 2 CO 3 The solution adjusts the pH of the reaction night to 8-9, extracts with dichloromethane (50mL×3) and separates the organic phase, then dried with anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography on silica gel to obtain a white solid 0.45
[0076] g, yield 61.5%, MS (ESI) m / z (%): 502.2[M+H] + .
[0077] 2. Synthesis of compound M3
[0078] Intermediate g3 (0.25g, 0.5mmol), intermediate b1 (0.093g, 0.65mmol), Pd(pph 3 ) 4 (0.029g, 0.025mmol), CuI (0.007g, 0.038mmol), DIPEA (0.19g, 1.5mmol), DMF (15mL), under nitrogen protection, react at 55℃ for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com