Salvianolic acid A salt complex and preparation method and application thereof
A technology of salvianolic acid and compound, applied in the fields of medicine and pharmacy, can solve the problems of difficulty in ensuring long-term stability, easy to be oxidized, poor stability of salvianolic acid A, etc. Myocardial ischemia-reperfusion injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
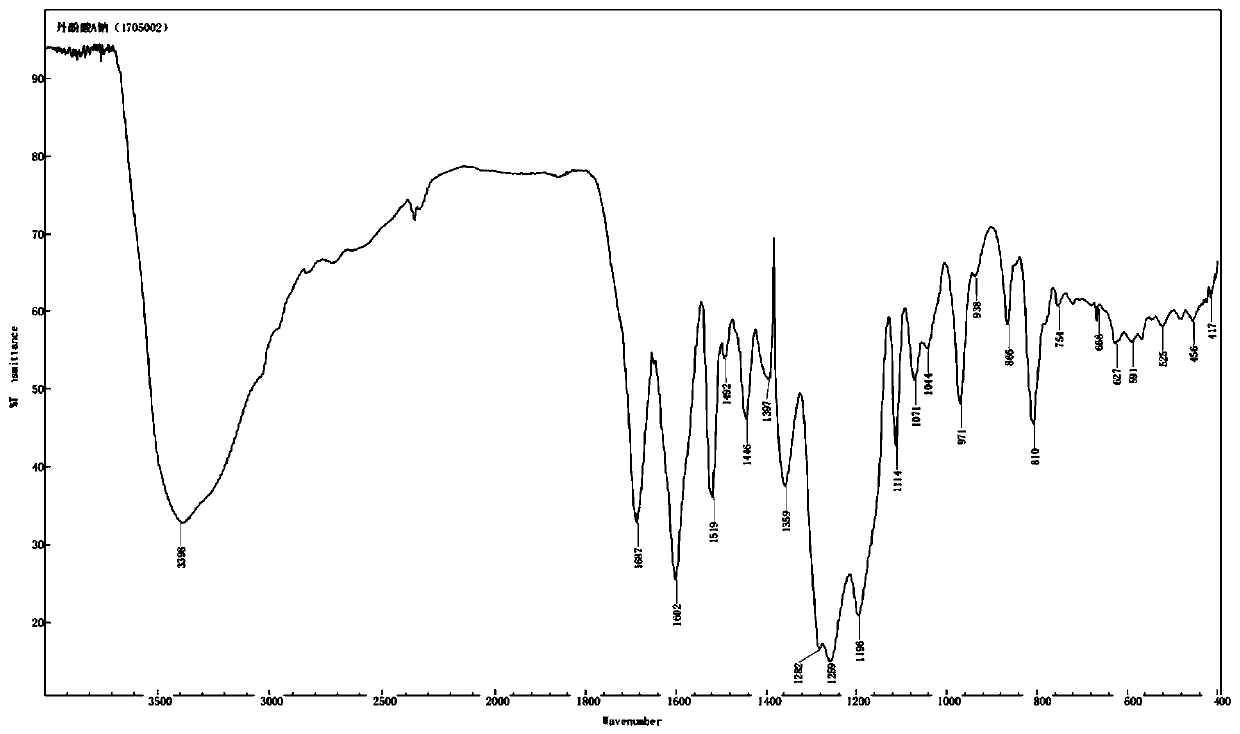
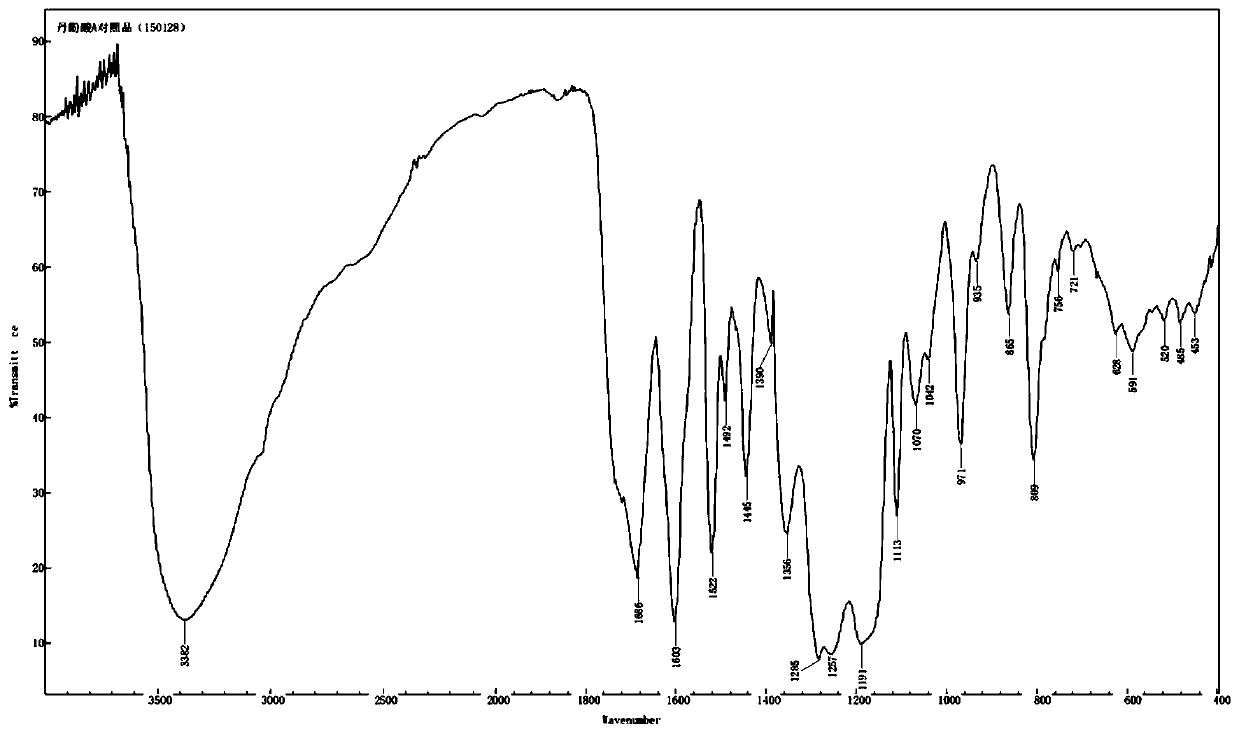
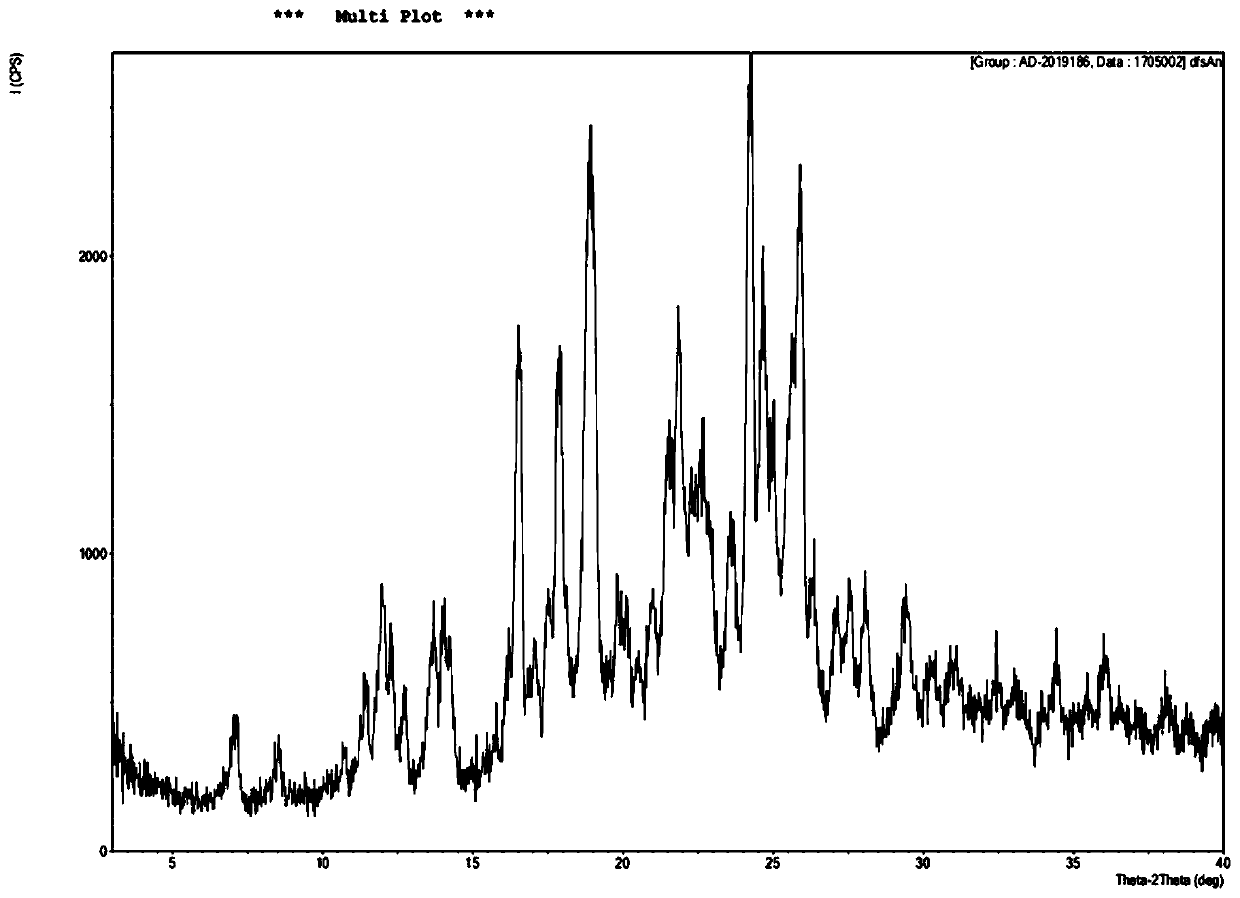
[0047] The preparation method of the salvianolic acid A salt compound of the present invention can refer to the method disclosed in CN 106748733A, and the preparation of the salvianolic acid A sodium compound is taken as an example below to illustrate:
[0048] Dissolve 1250 g of salvianolic acid A in 1010 mL of water, add 5% sodium hydroxide solution to adjust the pH to 3.6, filter, collect the precipitate, and dry under reduced pressure to obtain 767 g of salvianolic acid A sodium complex with a purity of 99.90%.
[0049] Other salvianolic acid A salt complexes can be prepared in the same way.
Embodiment 2
[0051] Accurately weigh an appropriate amount of the sodium salvianolic acid A compound prepared in Example 1, dissolve it in 5% glucose injection, and dilute it step by step to obtain different concentrations of medicinal solutions; take dog venous blood, and centrifuge at 800r / min after anticoagulation Separation of PRP for 10 minutes, separation of PPP by centrifugation at 2000r / min for 10 minutes, and 300 μL of each portion were distributed in colorimetric tubes. After confirming that PRP and PPP are suitable and available, 10 μL of liquid medicine were added, and after mixing, the final concentrations of the liquid medicines were respectively 1.41, 0.85, 0.51, 0.31, 0.18, 0.11, 0.07 and 0.04mg / mL, add 10μL 5% glucose injection in the control group; preheat the instrument for 5min, and detect platelets within 5min after adding 10μL ADP by Born nephelometric method The maximum aggregation rate (A max ), and calculate the aggregation inhibition rate according to formula 1. ...
Embodiment 3
[0058] Accurately weigh the appropriate amount of the sodium salvianolic acid A compound prepared in Example 1, dissolve it in distilled water and then dilute it with Krebs-Henseleit test solution to obtain medicinal solutions with different concentration gradients; healthy Wistar rats were taken, and quickly removed after anesthetized and killed. The upper part of the thoracic aorta strip was uniformly cut into 3-4mm vascular rings, suspended in the isolated perfusion device, inserted into two stainless steel hooks parallel to each other up and down, and the tension changes were recorded by the Pclab-UE biological signal acquisition system through the tension sensor. After equilibrating with K-H solution for 2 hours, add 40mmol / L KCl to shrink the vascular ring twice until the contraction is stable; pre-shrink the vascular ring with 40mmol / L KCl or 0.8μmol / L phenylephrine hydrochloride for 9min, add 0.002% For ascorbic acid, add different concentrations of salvianolic acid A s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com