Synthesis method of 4-cyano-3-(trifluoromethyl)benzene-1-sulfonyl chloride
A technology of trifluoromethyl and synthesis method, which is applied in the synthesis of 4-cyano-3-benzene-1-sulfonyl chloride and the field of pharmaceutical intermediates that inhibit the transient receptor potential A1 ion channel, and can solve environmental pollution compounds, Difficult to scale up production, high risk, etc., to achieve the effect of simple process, easy to obtain raw materials, and low risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
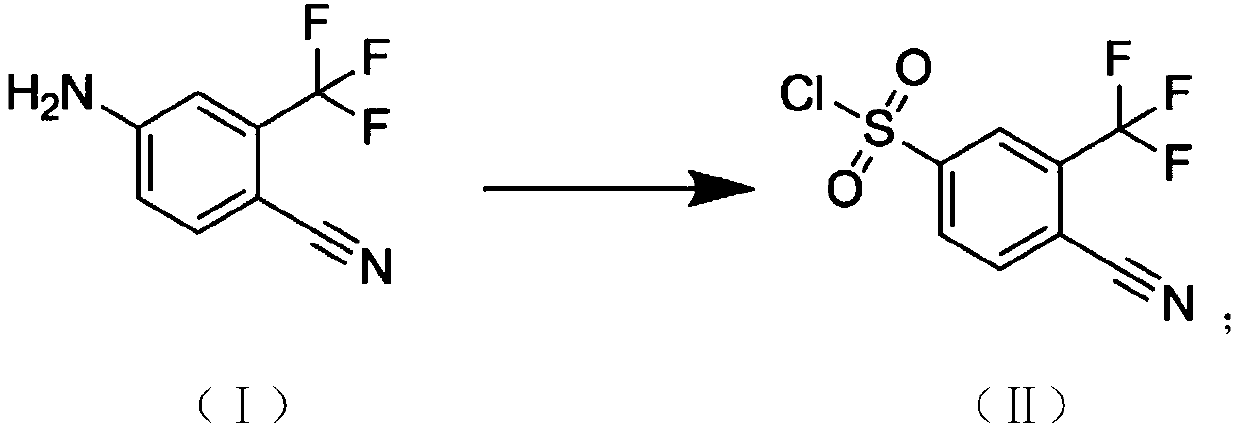
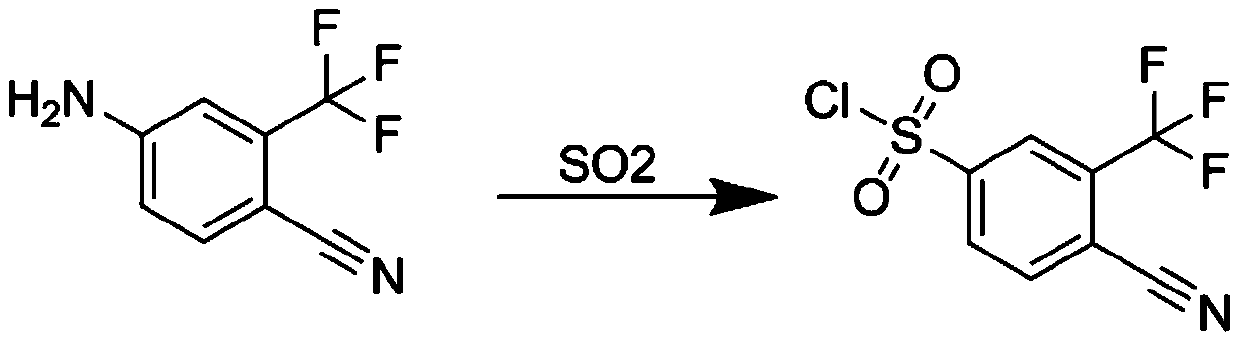
[0029] The reaction synthesis route of 4-cyano-3-(trifluoromethyl)benzene-1-sulfonyl chloride is as follows:
[0030]
[0031] The specific operation steps are:
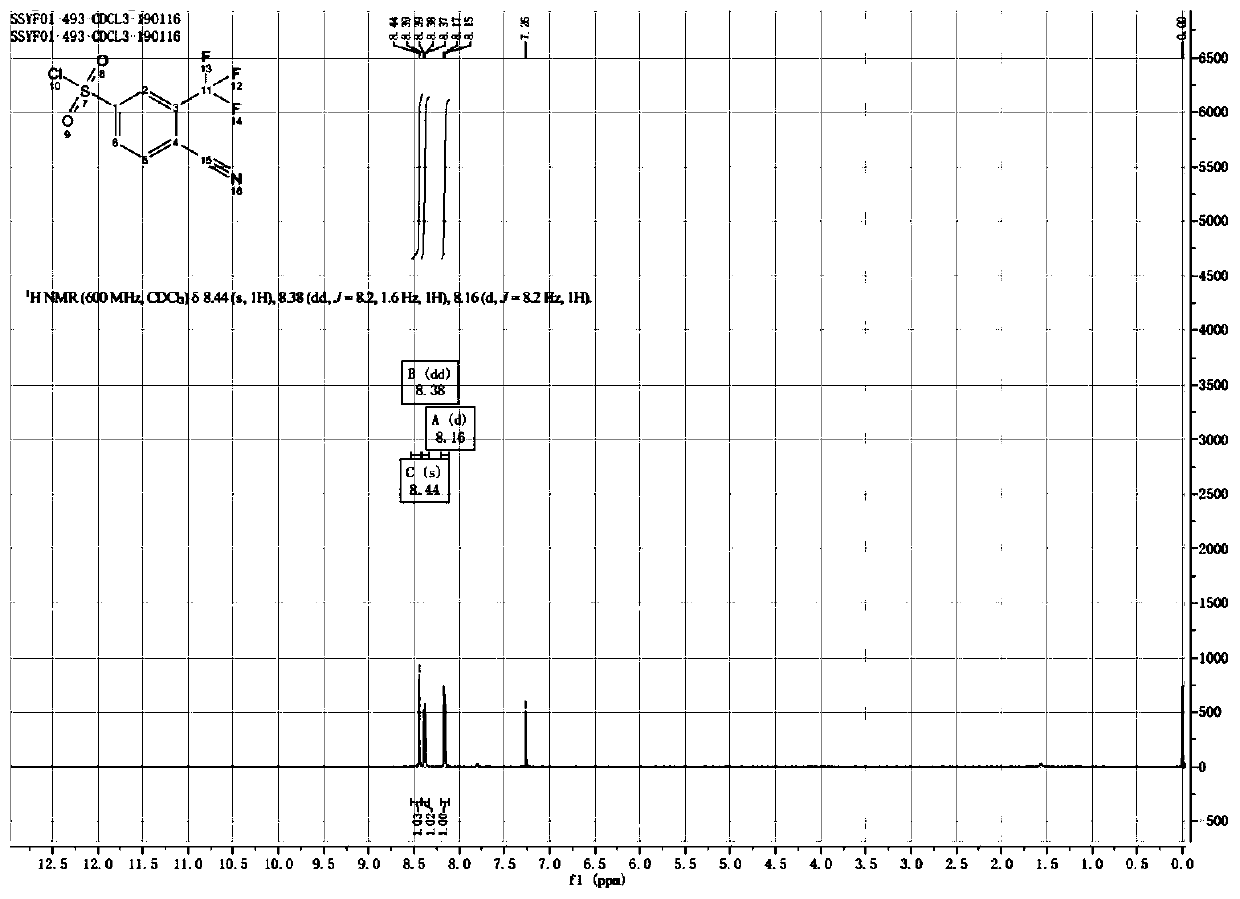
[0032] Anhydrous cuprous chloride (0.3g, 0.003mol, 0.011eq.) was added to 500ml of water, and 80ml of thionyl chloride was added dropwise in an ice-water bath, and overnight at rt, to obtain solution a; Compound 4-amino-2-(trifluoromethyl)benzonitrile (50.0g, 0.269mol, 1.0eq.) was added to 260ml of concentrated hydrochloric acid and 50ml of acetone in an ice-water bath, and sodium nitrite (20.4g , 0.296mol, 1.1eq.) was dissolved in 20ml of water, stirred at 0°C for 30 minutes to obtain reaction solution a; then reaction solution a was added dropwise to solution a, reacted at room temperature for 30 minutes, and TLC monitored whether the reaction was complete.
[0033] Reaction product treatment: flush with water, extract with EA, mix the sample and pass through the column to obtain 61.6 g of compound (II) with a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com