Method for preparing taxane natural product
A technology of natural products and taxanes, which is applied in the field of preparation of taxanes natural products, can solve the problems of low yield and achieve the effect of high yield and high availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
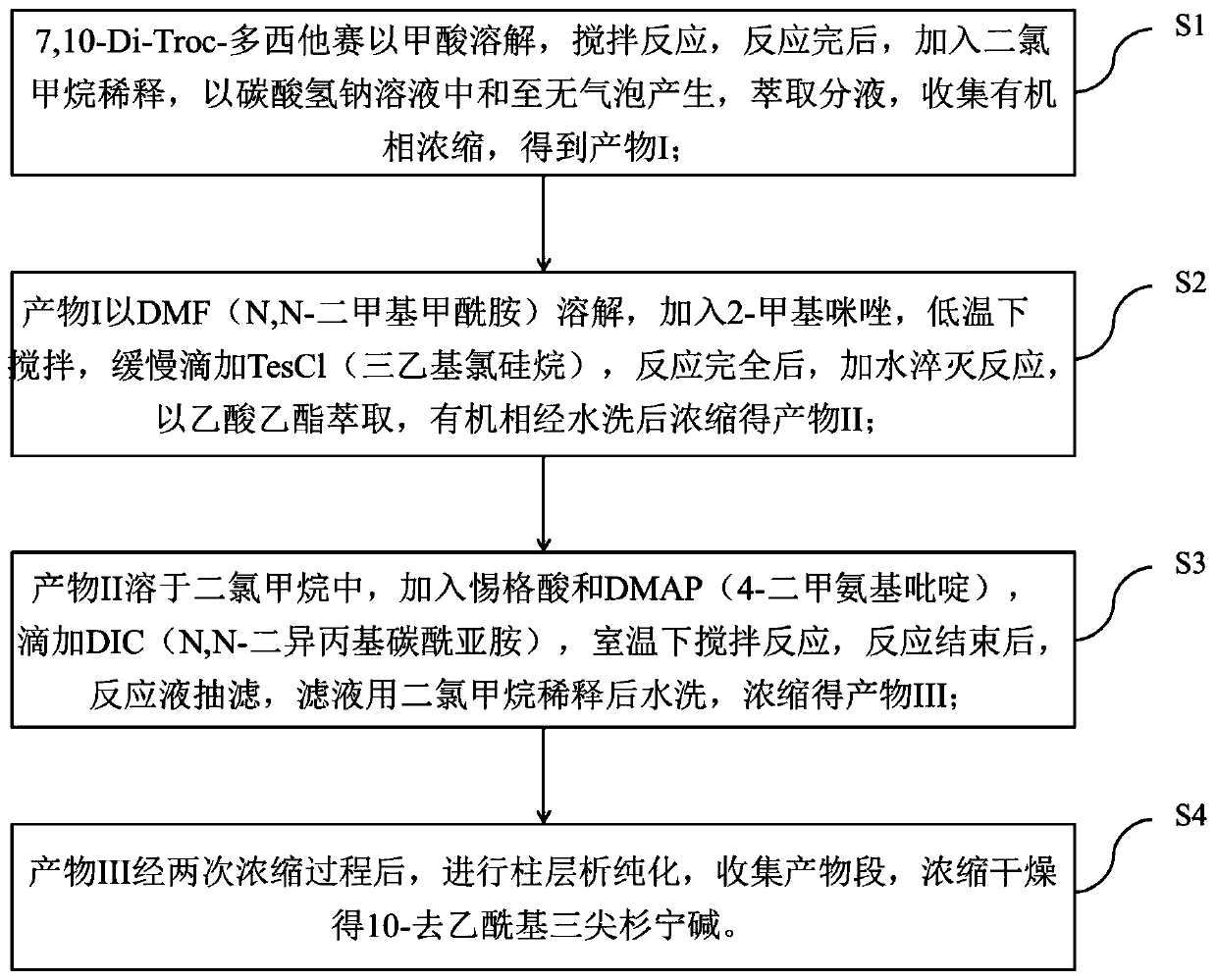
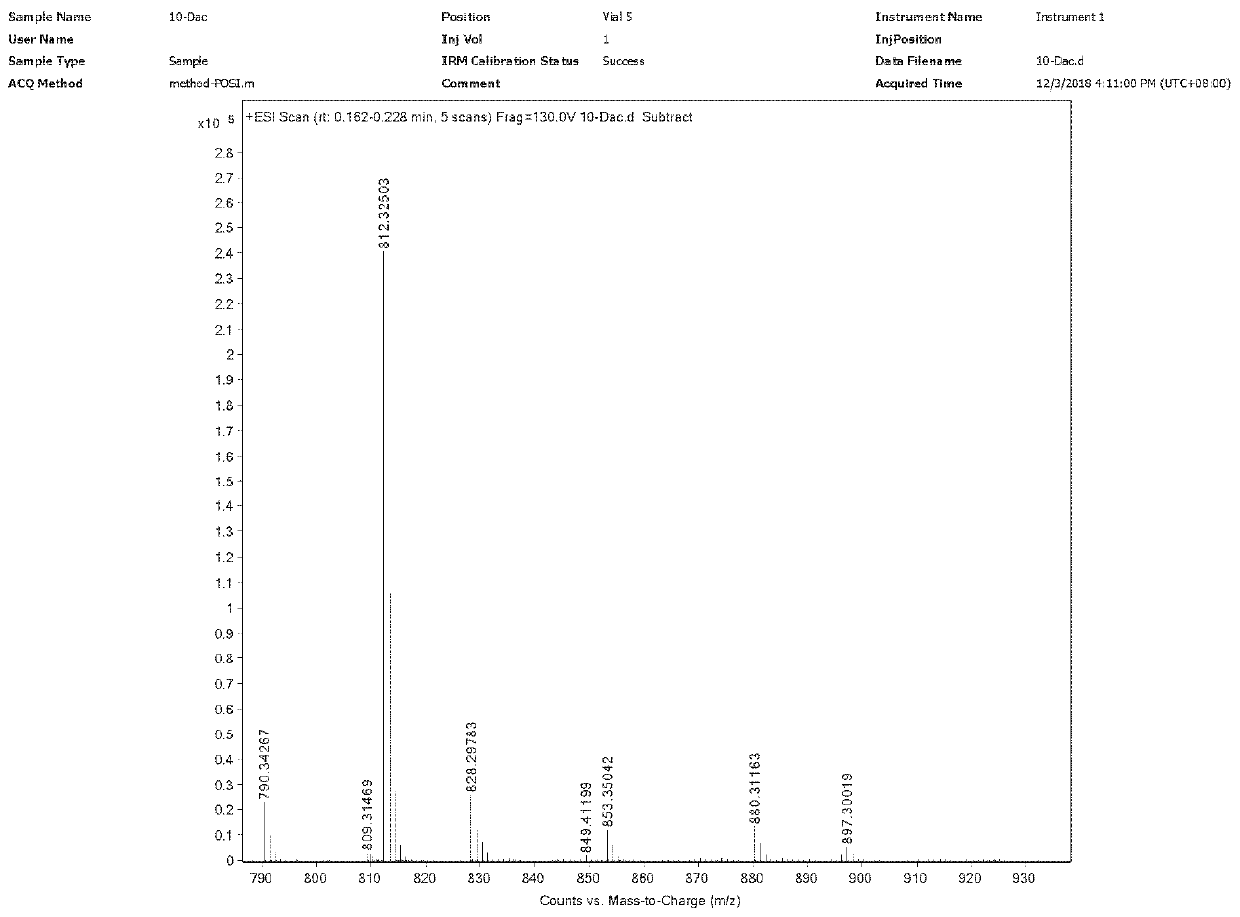
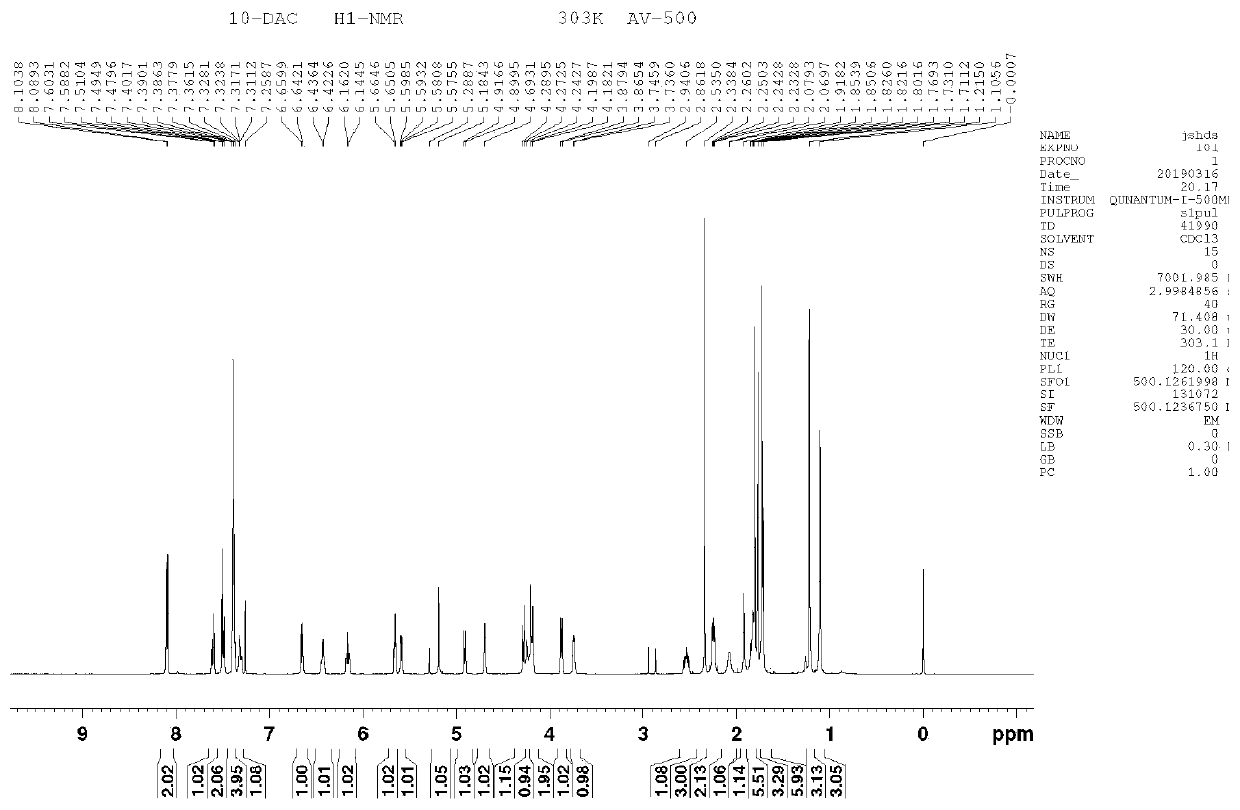
[0040] Step S1: Dissolve 7,10-Di-Troc-docetaxel (2.3g) in 14ml of formic acid and stir the reaction at room temperature (16°C). The sodium solution was neutralized until no bubbles were generated, the liquid was extracted and separated, and the organic phase was collected and concentrated to obtain 1.8 g of product I.
[0041] Step S2: Dissolve the product I (1.8g) in 6ml of DMF, add 2-methylimidazole (0.3g), stir in an ice bath to dissolve, slowly add TesCl (0.3g) dropwise, and continue the reaction for 60min after the dropwise addition , add 5ml of water to quench the reaction, extract with ethyl acetate, wash the organic phase three times with water, and concentrate to obtain 1.8g of product II.
[0042] Step S3: Dissolve the product II (1.8g) in 18ml of dichloromethane, add tigelic acid (0.18g) and DMAP (0.02g), add DIC (0.8ml) dropwise under stirring, and react at room temperature (15°C) 2h. After the reaction, the reaction liquid was suction filtered, the filtrate was ...
Embodiment 2
[0049] Step S1: Dissolve 7,10-Di-Troc-docetaxel (5.0g) in 50ml of formic acid, and stir the reaction at 25°C. After reacting for 1h, add 50ml of dichloromethane to dilute, and dilute with saturated sodium bicarbonate solution And until no bubbles are produced, the liquid is extracted and separated, and the organic phase is collected and concentrated to obtain 3.8g of product I.
[0050] Step S2: Dissolve the product I (3.8g) in 12ml of DMF, add 2-methylimidazole (0.87g), stir in an ice bath to dissolve, slowly add TesCl (1.0g) dropwise, and continue the reaction for 15min after the dropwise addition , add 5ml of water to quench the reaction, extract with ethyl acetate, wash the organic phase three times with water, and concentrate to obtain 3.9g of product II.
[0051] Step S3: Dissolve the product II (3.9g) in 40ml of dichloromethane, add tigelic acid (0.66g) and DMAP (0.19g), add DIC (2.3ml) dropwise under stirring, and react at 25°C for 1.5h. After the reaction, the reacti...
Embodiment 3
[0054] Step S1: Dissolve 7,10-Di-Troc-docetaxel (31.0g) in 240ml of formic acid and stir the reaction at room temperature (18°C). The sodium hydrogen solution was neutralized until no bubbles were generated, the liquid was extracted and separated, and the organic phase was collected and concentrated to obtain 25.3 g of product I.
[0055] Step S2: Dissolve the product I (25.3g) in 75ml DMF, add 2-methylimidazole (5.0g), stir in an ice bath to dissolve, slowly add TesCl (6.0g) dropwise, and continue the reaction for 45min after the dropwise addition , add 100ml of water to quench the reaction, extract with ethyl acetate, wash the organic phase with water three times, and concentrate to obtain 24.6g of product II.
[0056] Step S3: Dissolve the product II (24.6g) in 250ml of dichloromethane, add tigelic acid (3.5g) and DMAP (1.0g), add DIC (12ml) dropwise under stirring, and react at room temperature 20°C for 1h. After the reaction, the reaction liquid was suction filtered, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com