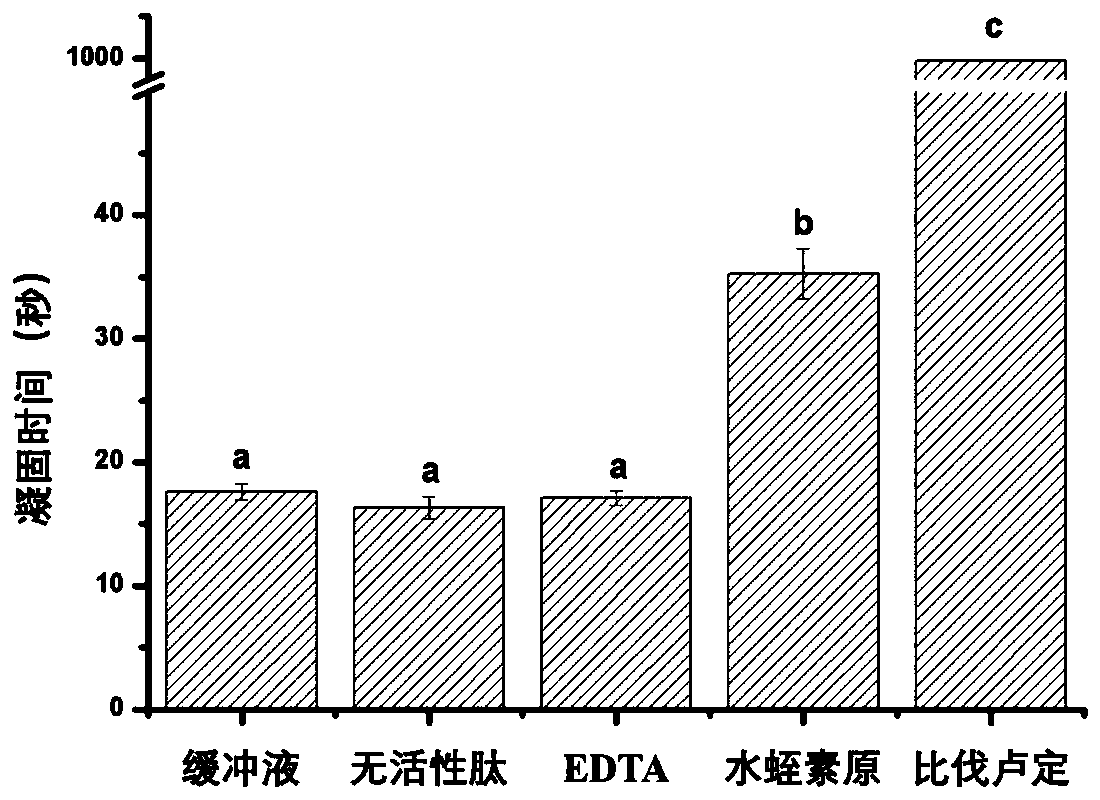
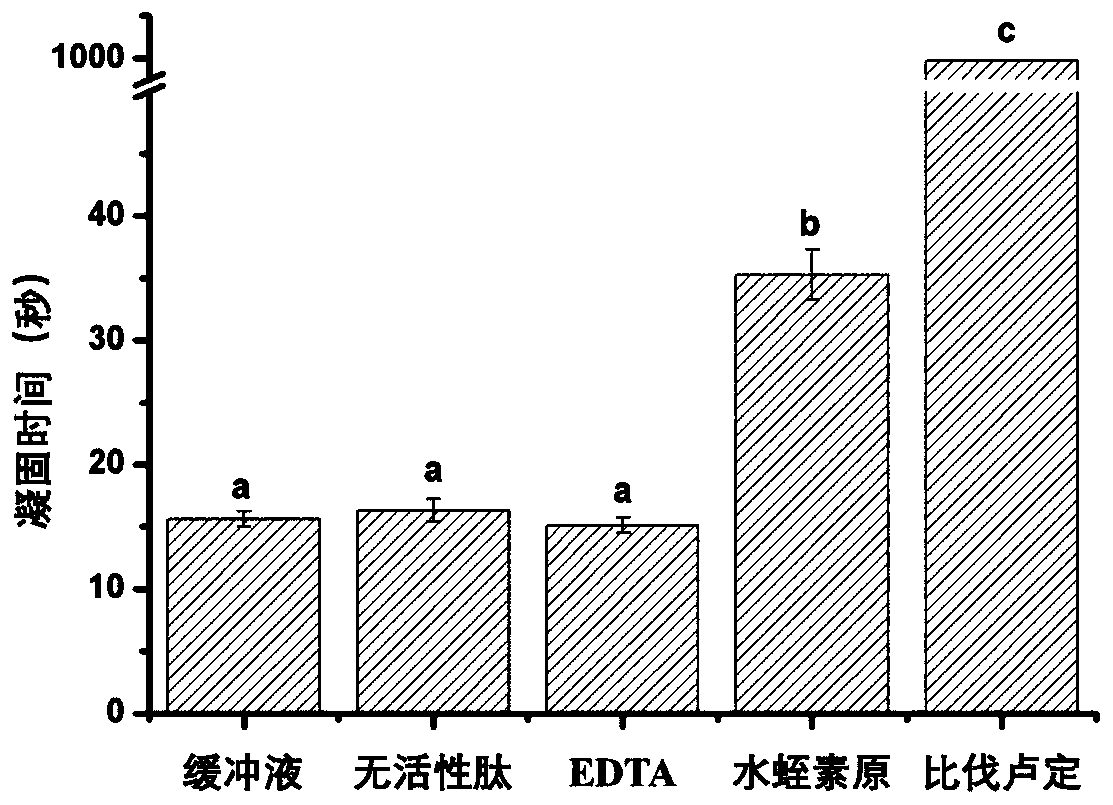
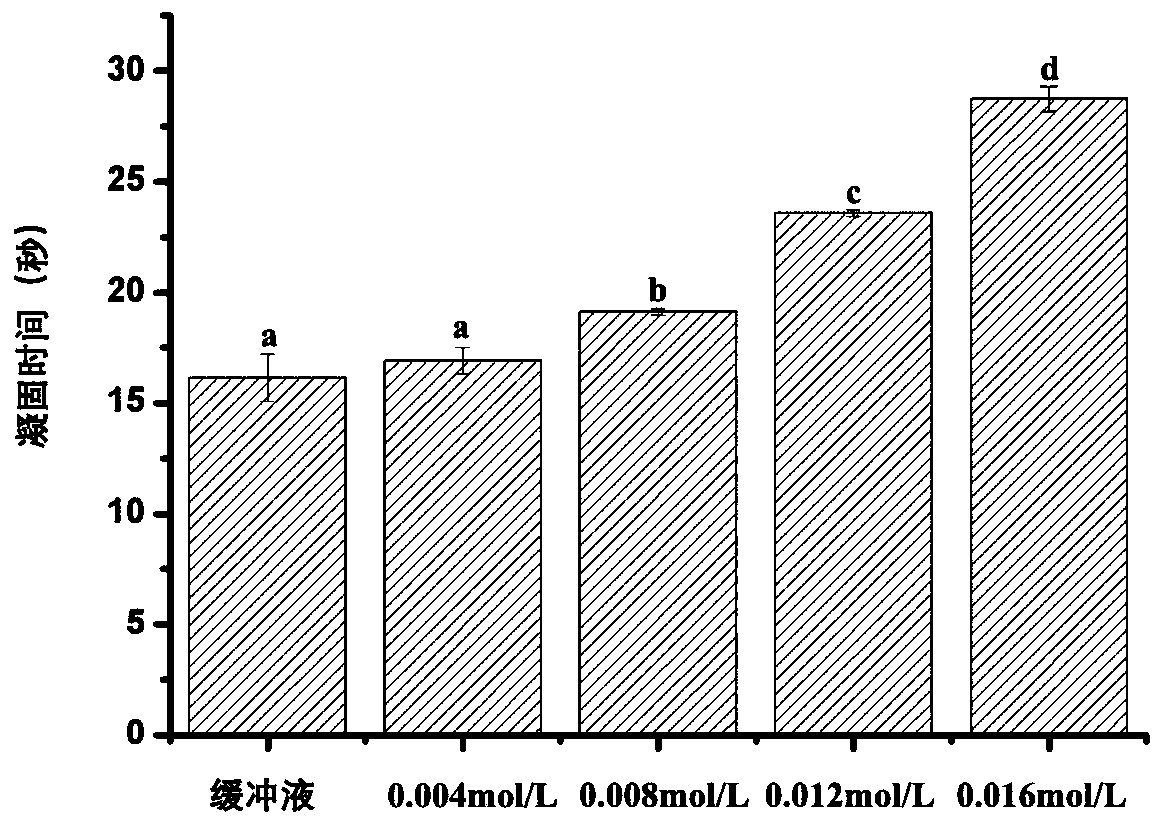
Determining method of thrombin inhibition activity
A technology of thrombin inhibitor and inhibitory activity, which is applied in the field of anticoagulation, can solve the problems of narrow application range and inability to consider the external site of thrombin, etc., achieve wide application range, optimize components and detection methods, and improve The effect of accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0034] Specific implementation mode 1: The detection method of thrombin inhibitor activity of this embodiment is implemented in the following steps:
[0035] 1. Configure buffer solution, configure imidazole concentration to be 0.05mol / L, sodium chloride concentration to be 0.1mol / L, calcium chloride concentration to be 0.002mol / L or Tris concentration to be 0.05mol / L, sodium chloride concentration to be 0.1mol / L, a buffer with a calcium chloride concentration of 0.002mol / L. Adjust the buffer pH to 7.4±0.05 with hydrochloric acid;
[0036] 2. Dissolve the inhibitor to be tested, fibrinogen, and thrombin with imidazole buffer. Place the fibrinogen solution at 37°C and the thrombin solution at 4°C for use;
[0037] 3. Add 200μL of fibrinogen solution and 100μL of inhibitor or buffer solution to the blood coagulation cup, incubate at 37°C, and then add 100μL of thrombin solution to the blood coagulation cup to detect the time required for fibrinogen to coagulate;
[0038] 4. Determine...
specific Embodiment approach 2
[0039] Specific embodiment two: This embodiment is different from the specific embodiment one in that the concentration of the fibrinogen solution in step two is 1 to 1.5 mg / mL. Other steps and parameters are the same as in the first embodiment.
specific Embodiment approach 3
[0040] Specific embodiment three: This embodiment is different from specific embodiment one or two in that the concentration of the thrombin solution in step two is 8-12 U / mL. Other steps and parameters are the same as those in the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com